Sleep related bruxism—comprehensive review of the literature based on a rare case presentation
Introduction
Sleep related bruxism (SRB) is an entity that has been a popular topic of debate in the dental and medical community in the recent several decades. While the American Academy of Sleep Medicine (AASM) has classified SRB as a sleep-related movement disorder (1), there is recent literature refuting this, and even classifying it as normal (2). Broadly, it is considered to differ from the wake bruxism by the fact that the latter is a “habit”, and therefore could be potentially controlled by interventional habit controlling methods (2). Attempts have been made to classify bruxism according to the various factors such as diurnal variation (wake and sleep-related), presence or absence of mandibular movements (static and dynamic), and etiology (primary and secondary) (3). It is of interest to the medical and dental community, both with reference to the probable abnormal nature of these movements during sleep, and due to the proposed destruction of the dentition, the temporomandibular joints (TMJs), and dental restorations. In addition, the observed association of SRB with other sleep related movement disorders, and possible medical comorbidities is of immense interest to the medical profession (4).
Sustained jaw clenching and/or repeated mandibular movements are hallmarks of SRB (5). The diagnostic criteria that are followed by various medical specialties vary vastly, and the current absolute lack of consensus amongst clinicians and researchers, has added to the existing confusion regarding the classification, diagnosis, and management modalities of this sleep related movement disorder. A diagnosis of SRB made based on patient, bed partner, or parental report is probably largely inaccurate. The dental clinician traditionally has resorted to such reports in combination with the observed changes in the dentition in an attempt to come to a diagnosis. An overnight polysomnography (PSG), although once considered the golden standard in the diagnosis of SRB, has been questioned in terms of the necessity for employment in a patient suspected of the same (1). The pathophysiology of SRB is unknown, with certain speculations and hypotheses floated in the literature. Recent attempts to include SRB as a “normal” or “protective” phenomenon has added more chaos to the already crowded arena of hypotheses (6). Consequently, there are no standardized management modalities either. It is the opinion of the authors that SRB should be looked at in the context of a sleep related movement disorder, and further succinct scientific studies are paramount in elucidating the mystery of this phenomenon. In this manuscript, we describe an interesting case of SRB, which has apparently traversed four generations. Here, we look at SRB as a probable movement disorder, shed light into the possible motor pathways, and discuss the genetic factors involved in SRB.
Methods
The search for this narrative review was performed between January 1st, 2021, and July 14th, 2022. The databases searched included PubMed, Google Scholar, Ovid, Embase, Science Direct, and textbooks on sleep disorders. The search terms included bruxism, sleep related bruxism, awake bruxism, sleep related movement disorder, pediatric bruxism, motor pathways of mastication, mandibular movements, rhythmic masticatory muscle activity (RMMA). The complete articles written in the English language were retrieved. Only articles published in English between 1970 and 2022 were included. Exclusion criteria included articles other than in English; articles published prior to 1970 and articles whose complete published form were not available.
The case
Twelve-year-old Indian fraternal twin girls presented along with parents with a chief complaint of “grinding her teeth in sleep at nighttime” in one of the twins.
History of presenting illness
According to a parental report, the affected child started SRB at an early age of 8 to 9 years. The child reportedly had bruxism episodes consistently “almost every night”. The episodes were reported as “bursts”. Most of the episodes were of a dynamic nature (“grinding”) with occasional static episodes (“clenching”). The child also complained about sporadic day time facial pain episodes and painful legs.
General features
The child appeared well nourished, well groomed, was very responsive to questions and cognizant. She was articulate and well oriented in terms of time and space. She was very expressive and devoid of anxiety at the first visit, although parents had given a history of anxious nature.
Medical history
Reported medical history was normal except for stomach hyperacidity and “pes planus”. Patient is on iron supplementation to manage her deficiency, which she has relatively poor compliance due to the side effects such as constipation.
Family history
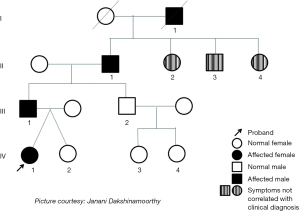
The twins’ father, grandfather and great grandfather have reported a history of SRB. The grandfather of the twins also had reported daytime bruxism. From the pedigree (see Figure 1), SRB seems to follow an autosomal dominant pattern of inheritance. It is observed that the proband (IV:1) has a father (III:1), grandfather (II:1) and a great grandfather (IV:1) who also have been diagnosed with SRB. It was also noted that the father’s brother (III:2) and his lineage did not show any clinical features of SRB. The siblings (II:2, II:3 and II:4) of the grandfather (II:1) were identified to have generalized attrition indicating the possibility of SRB. However, a diagnosis of SRB by report could not be verified in these siblings. The great grandfather (I:1) was reported to have a habit of grinding his teeth at night.
The affected twin child’s apparent psychological profile is very similar to her dad’s, in terms of common likes for apparel, type A personality, academic excellence and extrovertedness. They also have other common likes and dislikes. The common sleep trend between the father and the affected twin child includes somniloquy. One common factor among these four generations of individuals is hyperacidity/acid reflux. The unaffected twin does not suffer from acid reflux or hyperacidity. Both father and the affected twin child have a history of anxiety.
Dental examination
A comprehensive dental examination revealed normal soft and hard tissue structures, consistent with the twins’ age. There was no significant wear pattern observed in either child. TMJ and muscle examination were normal.
Investigations
An overnight PSG could not be ordered due to the fact that the child was not showing any other signs/symptoms of obstructive sleep apnea (OSA)/snoring; also due to safety concerns with the COVID-19 pandemic.
Diagnosis
The diagnosis of SRB in this case was entirely based on a parental report. There was no indication for a PSG due to the lack of evidence for snoring and OSA.
Review of literature
Definition of bruxism
SRB is defined by AASM as “an oral parafunction characterized by grinding or clenching of the teeth during sleep that is associated with an excessive (intense) sleep arousal activity” (1) and included as such in the International Classification of Sleep Disorders (3rd edition, 2014). There has been abundant discussion and confusion in the literature in defining and classifying bruxism. There are two distinct entities that have been identified namely, awake bruxism and SRB (7). Bruxism occurring in children is referred to generally as pediatric bruxism (PB). There is anecdotal evidence in the dental literature for possible genetic association with SRB. The American Academy of Orofacial Pain (AAOP, 2008) defines bruxism as “diurnal or nocturnal parafunctional activity including clenching, bracing, gnashing, and grinding of the teeth” (8). A recent international consensus statement does not consider SRB and awake bruxism to be movement disorders (2).
Primary/idiopathic sleep bruxism
This entity has been loosely defined in the literature, with considerable variations and controversies regarding its possible etiology. There has been strong suggestion in the literature of the role of the reticular activating system, and thereby the brain stem, contributing to increased motor and autonomic nervous system activity in SRB (9). There have been suggestions that SRB may be a result of improper processing of feedback from peripheral tissues such as teeth and the surrounding structures (10).
Secondary sleep bruxism
A class of drugs called selective serotonin reuptake inhibitors (SSRIs) have been implicated in the literature as etiologically related to secondary bruxism (11-16). Some recent articles, doing systematic reviews have concluded that a few medications such as duloxetine, venlafaxine, paroxetine, barbiturates may be related to higher risks of SRB (17). However, contrary to other literature, these authors reported lack of association of SRB with some SSRI medications such as citalopram, escitalopram, fluoxetine, sertraline. That said, it seems clear that drugs acting on the dopaminergic system promote awake bruxism in human patients (18,19).
Pediatric SRB
By definition, this entity occurs in children. There is no consensus in the current literature as to the diagnostic criteria or management of PB. Our experience is that explanations given to patients and parents by dental professionals (including teaching institutions) have not been based on rigorous scientific research. These explanations range from “because of mixed dentition”, “psychology” to “habit” to “intestinal worms/parasites” to “chronic abdominal distress”, and more. Recent literature has started looking at risk factors involved in bruxism in children. In these studies, risk factors were extremely varied, rendering a firm consensus challenging. Identified risk factors included male gender, genetics, anxiety, secondhand smoking, snoring, sleeplessness, and headache (20,21). Other studies have pointed to the significance of childhood stress in the etiopathogenesis of pediatric sleep bruxism (22).
Synonyms
Tooth grinding; clenching.
Epidemiology
The prevalence of PB is not well understood. Recent literatures including systematic reviews have reported the regional prevalence of PB (23,24). The highest reported prevalences in the descending order were Finland (40%), United States (37%), Brazil (35%), and Hong Kong (6%) (23,25). Studies show contradictory results, as to the association of bruxism with age (26-29). One of the reasons for the vastly varied findings in the prevalence rates is the differences and inconsistencies in diagnosis, primarily due to lack of universal diagnostic criteria for PB (25). Moreover, inconsistencies and errors in various parental report forms may be skewing the results (27,28,30). Studies from Brazil found high prevalence of PB in school children, with children having a habit of nail biting and biting objects at a possible higher risk of having PB (31,32). Gender differences in the prevalence of PB have been reported to be higher in males than females (30). Further, the same authors found a reduction in prevalence in both genders with age. Multiple studies over the last decade have resulted in diagonally opposite conclusions as to the incidence and prevalence, and association of PB with gender and age (27,28,30,33). A recent study shows the prevalence of PB as approximately 28% (34). The prevalence of sleep bruxism in adults was reported to be 9% in the general population, and as in children, adults also show no gender difference in SRB, and the prevalence reduces with age (35,36). A systematic review of epidemiology of adult bruxism shows rather inconclusive results. It was found to have a wide range of 8–31% for clinically identified bruxism, 22–31% for awake bruxism and approximately 13% for bruxism by report (36).
Etiology/pathophysiology
As the name indicates, the etiology of primary bruxism is not clearly understood. Secondary bruxism is associated with certain systemic diseases/or medications (37-39). The pathogenesis of bruxism is hypothesized to be multifactorial. Association of SRB with systemic disorders, sleep disordered breathing, restless leg syndrome, gastric reflux, and neurologic disorders, have been suggested (40,41). Although not clear in terms of association, increased urinary catecholamine levels were found in SRB patients, compared to normal individuals (42). The trigeminocardiac reflex (TCR) has recently been linked to various sleep disorders like OSA, SRB and rapid eye movement (REM) related sleep apnea (43).
Proposed motor pathways (in the context of movement disorder classification)
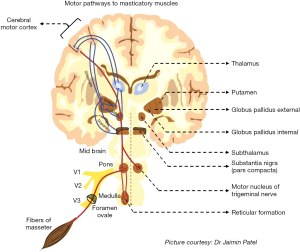
The motor pathways (see Figure 2) for volitional movements are widely considered to start from the pyramidal cells of the motor cortex (areas 4 & 6) (44). The role of the basal nuclei, their associated structures, and the related dopaminergic, glutamatergic and gabaergic pathways, in volitional movements, is well known (45,46). Diseases that cause abnormal functioning of these systems (such as parkinsonism) have been known to cause significant movement disorders such as dyskinesias, dystonias, athetosis, chorea and others (47,48). The critical role of the “direct and indirect” motor pathways in performing fine motor movements have also been elucidated (46,49). Consequently, the role of these centers, corticobulbar tracts and associated structures in normal mandibular movements, such as those involved in mastication, phonation, and swallowing are also well understood (50,51). However, the generation of mandibular movements that seemingly simulate these kinetics, such as that occur during SRB are not well understood (9,52). Similarly, the pathogenesis of REM sleep behavior disorders and associated abnormal movements are also poorly understood (53,54). Specifically, the lack of REM-related skeletal muscle atonia, occurring in REM-related bruxism is not understood as well (53).
A role of dopaminergic system in the pathophysiology of SRB has been suggested (55). This concept has been shown in animal models employing dopamine receptor agonists and antagonists (18,19). In line with this hypothesis, is the finding that striatal lesions of movement disorders such as Huntington’s disease show consistency with the level of bruxism in these patients (18,56). There have been suggestions in the literature that SRB-related movements may not originate from the motor cortex (2). With regards to the etiology of SRB, abnormalities in the central sensory motor processing have been proposed to be the cause of SRB (9). Central structures including, but not limited to, the locus coeruleus, the reticular formation, and the raphe magnum nuclei, have been shown to influence this type of sensory motor processing (57). The involvement of the brainstem neurons, by virtue of their effect on descending cortical pathways, has also been suggested in the pathogenesis of SRB (9).
Risk factors and associated conditions of SRB
Bruxism has been reported to be associated with the use of alcohol, tobacco and caffeine (38). SRB is associated with apnea and hypopnea related arousals, and the termination of apneas and hypopneas. OSA is considered a risk factor for SRB (58). Association of bruxism with such rare syndromes as Pallister-Killian syndrome and the associated genotypes have been reported (59). Other risk factors reported as associated with bruxism include poor sleep, prone sleep posture (60), snoring, and mouth breathing (34). Studies suggest that secondhand smoking and sleep disturbances have the strongest association with SRB (61). The same authors found weak associations of occlusal variations, with SRB (62). Further, they also found the history of childhood bruxism, gastro-esophageal reflux disease (GERD), and genetic polymorphisms to be the most important risk factors linked with adult SRB (63). Articles have also looked at changes in the upper airway as being mostly associated with pediatric SRB (64-66). Improper sleep hygiene and a loud external environment have been proposed to be associated with SRB (64,67). Male children with the habit of object biting, nail biting and lip biting have been reported to have a strong association with severe SRB (23).
Studies show an association with psychological factors such as stress, anxiety, and personality traits (68). Some studies demonstrate the association of bruxism with sleep disorders (69). Recent studies do show an association of SRB to chronic stress or sleep quality (70). There have been isolated articles in the literature that cast a doubt on the association between pediatric SRB and anxiety traits (71). Older dental literature referred to the association of bruxism with occlusal abnormalities (72). Literature and research over the past few decades have successfully debunked this notion (57,73,74). Literature suggests that sensitivity to stress might be more important as an association, and predictive of SRB, than anxiety itself (69). There are contradicting reports of a possible association of SRB with OSA. OSA and SRB share common clinical features, and OSA therapy does improve SRB variables (75,76). The association of SRB with limb movements during sleep has been well reported (77,78). The same literature hypothesizes that the control of SRB episodes may be related to motor pathways of jaw movements and limb movements. Also, studies have shown a strong association of SRB with slow movements of the eyes, as that occurs in the N1 and N2 stages of sleep (79).
Genetics of SRB
Recent studies demonstrate a robust relationship between SRB and genetics (35). A positive association with genes that code for the serotonin and dopamine receptors, 5-HTR2A and DRD1 respectively, were found (80). In a recent study, there was a genomic association of the 5-HT2A receptor nucleotide as a risk factor for sleep bruxism (81). Other articles have alluded to the association of 5-HTR2A polymorphism as a potential risk factor for SRB (82). A recent study suggested an association between bruxism and the enzyme matrix metalloproteinase 9 (MMP-9) in both adults and children (83). Single nucleotide polymorphisms in dopamine receptors were found to be associated with bruxism and were also associated with circadian phenotypes in children (84). Recent studies suggest that bruxism is partly genetically determined, demonstrating that the chance of bruxism in children changes proportionately as the percentage of parental bruxers increases (85).
The Online Mendelian Inheritance in Man (OMIM) website classifies sleep bruxism along with parasomnias, and uses the alternative title “facio-mandibular myoclonus” (OMIM 606840). There is moderate evidence for the role of genetics as a risk factor in the causation of SRB (63,86). Studies demonstrate that neurotransmitters in the central nervous system (CNS) and their associated genes may be factors in the pathophysiology of SRB (57,68,81). The serotonin receptor encoding gene HTR2A, catechol-O-methyltransferase (COMT), and the dopamine receptor gene (DRD1) have been shown to be associated with SRB (80). The same study indicates increased bruxism related episodes that were related to the gene HTR2A homozygous mutation, as opposed to heterozygous mutation on rs6313. A possible genetic contribution as etiologically related to primary sleep bruxism was suggested in these recent studies.
Clinical features of SRB
Some of the literature describes how pain is associated with SRB and parafunctional habits (87). It has also been reported in the literature that pain or “tense feeling” is associated with SRB, and other distinct features such as TMJ noises, stress and smoking being associated with awake bruxism (88). Fatigue of the muscles of mastication with SRB in children/adolescents (self-reported) has been reported in the literature (89). Other orofacial findings in pediatric SRB include ridges on the side of the tongue (90) and on cheek mucosa (91); dental attrition (9); hypersalivation (92) and also hypertrophic masseters (9). TMJ clicks and noises are associated with severe SRB, especially in adolescence (93). Non-carious lesions show association with SRB, compared to controls (94). Features like somniloquy and hypersalivation have been reported to be associated with SRB in children (95). Studies demonstrate a higher prevalence of degenerative TMJ disorders with SRB (96). Anecdotal reports describe the effects of SRB on the dental structures, such as attrition, loss of tooth structure, chipping of enamel, increased tooth sensitivity, and “enamel craze lines”, tooth fractures, dental restoration failures, and dental implant failures (97-100).
Diagnosis
The gold standard for qualitative and quantitative diagnosis of SRB is an overnight PSG (101,102). The latest AASM guidelines do not require a PSG for diagnosis of SRB unless there is other clinical evidence of sleep disordered breathing (103). Portable devices may be useful for the diagnosis of SRB in both research and clinical settings (104), however, further research is needed. Novel techniques include the use of sensors in bruxism appliances for monitoring SRB (105). This is an important area in the field as studies demonstrate a diagnostic discrepancy between parental reports compared to PSG (69,106).
Management
A recent systematic review found efficacy with all types of oral appliances, specifically mandibular advancement devices in reducing SRB (107,108). The same authors also report moderate efficacy for botulinum toxin, clonazepam, clonidine, and electrical stimuli of the masseter muscles.
Rapid palatal expansion
Rapid palatal expansion has been proposed as a therapy for pediatric bruxers and showed moderate reduction in the RMMA (Rhythmic Masticatory Muscle Activity) (109).
Medications
Management of concomitant stomach hyperacidity associated with SRB, has shown to be effective in reducing the frequency of RMMA and muscle activity bursts (110). The lack of effectiveness of medications in managing SRB was found in some of the literature (111). The use of botulinum toxin has been proposed as a therapy for SRB, with relatively good short-term results (112,113). However, it is not clear if the botulinum toxin therapy favorably affects the central motor pattern generator of SRB. There is recent evidence in the literature of moderately beneficial use of homeopathic medications rendering positive effects in the management of SRB (114,115). For the management of drug-induced (secondary) bruxism, discontinuation or substitution of the offending drug and possible addition of buspirone has been suggested (15).
Occlusal splints
There is controversy regarding the long-term efficacy of occlusal splints in managing SRB. While it is clear that the splint could act to protect the dentition/restorations, its role in managing SRB activity itself is unclear. The lack of consensus stems from factors such as variation in the definition and diagnostic criteria, and lack of high quality randomized prospective clinical trials. Other articles also conclude by casting doubt on any substantial benefit of splints in the management of SRB regardless of the appliance design or material (116).
Some of the literature talks about the advantage of intermittent rather than continuous use of dental splints, reducing SRB activity better than the latter (102,117). It should be borne in mind that these appliances do have the potential to induce dental occlusal changes and other TMJ changes upon long term use. A Cochrane systematic review concluded that there is no evidence of any long-term efficacy of splints in SRB management, benefiting only in the protection of dental structures (118).
Psycho-social interventions
Psychotherapeutic modalities including biofeedback and cognitive behavior therapy (CBT) have been investigated as management methods for SRB. By and large, CBT is considered one of the most effective methods for conservative management of SRB (119).
Case discussion in the context of literature
As per the parental report, the child started SRB at the age of eight to nine years. This finding is consistent with recent studies on pediatric SRB (31,32). The patient had bruxism episodes that were “grinding” and occasionally “clenching” in nature. This finding is consistent with the definition of bruxism by AASM (1). The child complained of sporadic day time facial pain episodes and painful legs. Some literature describes the association of pain with SRB and parafunctional habits (87). As alluded to earlier, pain/“tense feeling” is associated with SRB (88). Also, association of fatigue of jaw muscles with SRB in children/adolescents (self-reported) has been reported in the literature (89). TMJ clicks/noises have been reported to be associated with relatively severe SRB, especially in adolescence (93).
Contrary to some reports (20), the affected twin in our case was articulate and well oriented in terms of time and space. She was very expressive and devoid of anxiety at the first visit, although parents had given a history of anxious nature. Our case findings were not consistent with a few prior studies (60,120), where SRB was associated with tooth caries, dental attrition, malocclusion and respiratory issues, as well as economic status and use of pacifiers. Jaw muscle fatigue was consistent with the literature (89). The child was not having any difficulties in mouth opening, which was not consistent with literature (121). Breathing problems, usually reported in the literature as associated with SRB (34,122), were not present in our case. Sleep disturbances presented the strongest association with SRB (62), but not in our case. There are several features in the medical history of this child that are both consistent and inconsistent with the established literature. The comparison of the medical history features of the present case to the existing literature is given in Table 1.
Table 1
| Features | Current case | As per literature |
|---|---|---|
| Age of onset | 8–9 years old | 8–10 years (31,32) |
| Episodes | Bursts/daily | Bursts (1) |
| Dynamic bruxism | Dynamic bruxism (1,57) | |
| Facial and leg pain | Present | Facial pain (57,89) |
| Leg pain (87) | ||
| Stress/anxiety | Anxious | Stress (22,69,70) |
| Anxiety (68) | ||
| Pes planus | Present | Not found in literature |
| Joint hypermobility | Absent | Not found in literature |
| Stomach hyperacidity | Present | GERD (53,63,123) |
| Iron deficiency | Present | Conflicting reports on association of Ferritin and SRB (124) |
| Family history of bruxism | Present | Reported (92,125) |
| Personality trait | Talkative, extroverted and Type A personality | Reported (68,126) |
| Concomitant sleep disorders | Somniloquy | Somniloquy (57) |
| Hypersalivation (91) | ||
| OSA (58,76,127) | ||
| Tooth fracture or wear | Absent | Reported (97,128,129) |
| TMJ pain | Absent | Reported (87,130,131) |
| Polysomnography | Not done | Most confirmatory test for diagnosis of SRB (101,102) |
| Bowel habits | Constipation | Not found in literature |
| Socio economic status | Upper strata of society | Upper strata of society (28,132-134) |
TMJ, temporomandibular joint; GERD, gastro-esophageal reflux disease; SRB, sleep related bruxism; OSA, obstructive sleep apnea.
Conclusions
SRB is a sleep related movement disorder which is centrally generated, and may have a significant genetic component. The pathophysiology remains largely unknown. The theories and apparent consensus on this phenomenon seem to be largely inadequate to explain the various aspects of this entity. Dental occlusion has been ruled out as a factor in the pathogenesis of this condition. High quality randomized controlled trials are needed to evaluate the various management modalities that currently exist for SRB.
Acknowledgments
The authors acknowledge the contribution of Swetha Kannan towards the digitalization of Figure 2.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Frontiers of Oral and Maxillofacial Medicine for the series “Orofacial Pain”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-102/coif). The series “Orofacial Pain” was commissioned by the editorial office without any funding or sponsorship. DCT served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014;146:1387-94. [Crossref] [PubMed]
- Lobbezoo F, Ahlberg J, Raphael KG, et al. International consensus on the assessment of bruxism: Report of a work in progress. J Oral Rehabil 2018;45:837-44. [Crossref] [PubMed]
- Paesani DAAM. Bruxism: theory and practice. London; Chicago: Quintessence Pub., 2010.
- Kuang B, Li D, Lobbezoo F, et al. Associations between sleep bruxism and other sleep-related disorders in adults: a systematic review. Sleep Med 2022;89:31-47. [Crossref] [PubMed]
- Okura K, Shigemoto S, Suzuki Y, et al. Mandibular movement during sleep bruxism associated with current tooth attrition. J Prosthodont Res 2017;61:87-95. [Crossref] [PubMed]
- Manfredini D, Guarda-Nardini L, Marchese-Ragona R, et al. Theories on possible temporal relationships between sleep bruxism and obstructive sleep apnea events. An expert opinion. Sleep Breath 2015;19:1459-65. [Crossref] [PubMed]
- Cruz-Fierro N, Martínez-Fierro M, Cerda-Flores RM, et al. The phenotype, psychotype and genotype of bruxism. Biomed Rep 2018;8:264-8. [Crossref] [PubMed]
- American Academy of Orofacial Pain. Orofacial pain: guidelines for assessment, diagnosis, and management. Chicago: Quintessence Publ., 2008.
- Lavigne GJ, Huynh N, Kato T, et al. Genesis of sleep bruxism: motor and autonomic-cardiac interactions. Arch Oral Biol 2007;52:381-4. [Crossref] [PubMed]
- Laine CM, Yavuz ŞU, D'Amico JM, et al. Jaw tremor as a physiological biomarker of bruxism. Clin Neurophysiol 2015;126:1746-53. [Crossref] [PubMed]
- Milanlioglu A. Paroxetine-induced severe sleep bruxism successfully treated with buspirone. Clinics (Sao Paulo) 2012;67:191-2. [Crossref] [PubMed]
- Akbaş B, Bilgiç A. Fluoxetine-Induced Sleep Bruxism Rapidly Treated With Once-Nightly Dosing of Buspirone in a 6-Year-Old Girl. Clin Neuropharmacol 2018;41:197-8. [Crossref] [PubMed]
- Çolak Sivri R, Akça ÖF. Buspirone in the Treatment of Fluoxetine-Induced Sleep Bruxism. J Child Adolesc Psychopharmacol 2016;26:762-3. [Crossref] [PubMed]
- Zandifar A, Mohammadi MR, Badrfam R. Low-Dose Quetiapine in the Treatment of SSRI-Induced Bruxism and Mandibular Dystonia: Case Series. Iran J Psychiatry 2018;13:227-9. [PubMed]
- Garrett AR, Hawley JS. SSRI-associated bruxism: A systematic review of published case reports. Neurol Clin Pract 2018;8:135-41. [Crossref] [PubMed]
- Uvais NA, Sreeraj VS, Sathish Kumar SV. Sertraline induced mandibular dystonia and bruxism. J Family Med Prim Care 2016;5:882-4. [Crossref] [PubMed]
- Melo G, Dutra KL, Rodrigues Filho R, et al. Association between psychotropic medications and presence of sleep bruxism: A systematic review. J Oral Rehabil 2018;45:545-54. [Crossref] [PubMed]
- Ella B, Ghorayeb I, Burbaud P, et al. Bruxism in Movement Disorders: A Comprehensive Review. J Prosthodont 2017;26:599-605. [Crossref] [PubMed]
- Winocur E, Gavish A, Voikovitch M, et al. Drugs and bruxism: a critical review. J Orofac Pain 2003;17:99-111. [PubMed]
- Ribeiro MB, Manfredini D, Tavares-Silva C, et al. Association of possible sleep bruxism in children with different chronotype profiles and sleep characteristics. Chronobiol Int 2018;35:633-42. [Crossref] [PubMed]
- Guo H, Wang T, Niu X, et al. The risk factors related to bruxism in children: A systematic review and meta-analysis. Arch Oral Biol 2018;86:18-34. [Crossref] [PubMed]
- Drumond CL, Paiva SM, Vieira-Andrade RG, et al. Do family functioning and mothers' and children's stress increase the odds of probable sleep bruxism among schoolchildren? A case control study. Clin Oral Investig 2020;24:1025-33. [Crossref] [PubMed]
- Soares JP, Giacomin A, Cardoso M, et al. Association of gender, oral habits, and poor sleep quality with possible sleep bruxism in schoolchildren. Braz Oral Res 2020;34:e019. [Crossref] [PubMed]
- Manfredini D, Restrepo C, Diaz-Serrano K, et al. Prevalence of sleep bruxism in children: a systematic review of the literature. J Oral Rehabil 2013;40:631-42. [Crossref] [PubMed]
- Machado E, Dal-Fabbro C, Cunali PA, et al. Prevalence of sleep bruxism in children: a systematic review. Dental Press J Orthod 2014;19:54-61. [Crossref] [PubMed]
- Massignan C, de Alencar NA, Soares JP, et al. Poor sleep quality and prevalence of probable sleep bruxism in primary and mixed dentitions: a cross-sectional study. Sleep Breath 2019;23:935-41. [Crossref] [PubMed]
- Insana SP, Gozal D, McNeil DW, et al. Community based study of sleep bruxism during early childhood. Sleep Med 2013;14:183-8. [Crossref] [PubMed]
- Serra-Negra JM, Paiva SM, Seabra AP, et al. Prevalence of sleep bruxism in a group of Brazilian schoolchildren. Eur Arch Paediatr Dent 2010;11:192-5. [Crossref] [PubMed]
- Liu X, Ma Y, Wang Y, et al. Brief report: An epidemiologic survey of the prevalence of sleep disorders among children 2 to 12 years old in Beijing, China. Pediatrics 2005;115:266-8. [Crossref] [PubMed]
- Lam MH, Zhang J, Li AM, et al. A community study of sleep bruxism in Hong Kong children: association with comorbid sleep disorders and neurobehavioral consequences. Sleep Med 2011;12:641-5. [Crossref] [PubMed]
- Drumond CL, Ramos-Jorge J, Vieira-Andrade RG, et al. Prevalence of probable sleep bruxism and associated factors in Brazilian schoolchildren. Int J Paediatr Dent 2018; [Crossref] [PubMed]
- Serra-Negra JM, Paiva SM, Auad SM, et al. Signs, symptoms, parafunctions and associated factors of parent-reported sleep bruxism in children: a case-control study. Braz Dent J 2012;23:746-52. [Crossref] [PubMed]
- Fonseca CM, dos Santos MB, Consani RL, et al. Incidence of sleep bruxism among children in Itanhandu, Brazil. Sleep Breath 2011;15:215-20. [Crossref] [PubMed]
- Lamenha Lins RM, Cavalcanti Campêlo MC, Mello Figueiredo L, et al. Probable Sleep Bruxism in Children and its Relationship with Harmful Oral Habits, Type of Crossbite and Oral Breathing. J Clin Pediatr Dent 2020;44:66-9. [Crossref] [PubMed]
- Khoury S, Carra MC, Huynh N, et al. Sleep Bruxism-Tooth Grinding Prevalence, Characteristics and Familial Aggregation: A Large Cross-Sectional Survey and Polysomnographic Validation. Sleep 2016;39:2049-56. [Crossref] [PubMed]
- Manfredini D, Winocur E, Guarda-Nardini L, et al. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain 2013;27:99-110. [Crossref] [PubMed]
- Teoh L, Moses G. Drug-induced bruxism. Aust Prescr 2019;42:121. [Crossref] [PubMed]
- Bertazzo-Silveira E, Kruger CM, Porto De Toledo I, et al. Association between sleep bruxism and alcohol, caffeine, tobacco, and drug abuse: A systematic review. J Am Dent Assoc 2016;147:859-866.e4. [Crossref] [PubMed]
- Falisi G, Rastelli C, Panti F, et al. Psychotropic drugs and bruxism. Expert Opin Drug Saf 2014;13:1319-26. [Crossref] [PubMed]
- Wieckiewicz M, Winocur E. Editorial: Orofacial Pain, Bruxism, and Sleep. Front Neurol 2020;11:555. [Crossref] [PubMed]
- Carra MC, Huynh N, Lavigne G. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am 2012;56:387-413. [Crossref] [PubMed]
- Zhong Z, Xu M, Zou X, et al. Changes in heart rate related to rhythmic masticatory muscle activities and limb movements in patients with sleep bruxism. J Oral Rehabil 2020;47:170-9. [Crossref] [PubMed]
- Bindu B, Singh GP, Chowdhury T, et al. Rhinitis and sleep disorders: The trigeminocardiac reflex link? Med Hypotheses 2017;103:96-9. [Crossref] [PubMed]
- Emos MC, Agarwal S. Neuroanatomy, Upper Motor Neuron Lesion. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC., 2021.
- O'Gorman Tuura RL, Baumann CR, Baumann-Vogel H. Beyond Dopamine: GABA, Glutamate, and the Axial Symptoms of Parkinson Disease. Front Neurol 2018;9:806. [Crossref] [PubMed]
- Calabresi P, Picconi B, Tozzi A, et al. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 2014;17:1022-30. [Crossref] [PubMed]
- Sanders RD, Gillig PM. Extrapyramidal examinations in psychiatry. Innov Clin Neurosci 2012;9:10-6. [PubMed]
- Ellrichmann G, Russ H, Müller T. Dyskinesia in Parkinson's disease--major clinical features, aetiology, therapy. Fortschr Neurol Psychiatr 2007;75:387-96. [Crossref] [PubMed]
- Isa T, Kinoshita M, Nishimura Y. Role of Direct vs. Indirect Pathways from the Motor Cortex to Spinal Motoneurons in the Control of Hand Dexterity. Front Neurol 2013;4:191. [Crossref] [PubMed]
- Bhardwaj N, Yadala S. Neuroanatomy, Corticobulbar Tract. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC., 2021.
- Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. The motor cortex and facial expression: new insights from neuroscience. Neurologist 2004;10:235-49. [Crossref] [PubMed]
- Lavigne GJ, Kato T, Kolta A, et al. Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med 2003;14:30-46. [Crossref] [PubMed]
- Chan PC, Lee HH, Hong CT, et al. REM Sleep Behavior Disorder (RBD) in Dementia with Lewy Bodies (DLB). Behav Neurol 2018;2018:9421098. [Crossref] [PubMed]
- Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 2010;1184:15-54. [Crossref] [PubMed]
- Lobbezoo F, Soucy JP, Montplaisir JY, et al. Striatal D2 receptor binding in sleep bruxism: a controlled study with iodine-123-iodobenzamide and single-photon-emission computed tomography. J Dent Res 1996;75:1804-10. [Crossref] [PubMed]
- Tan EK, Jankovic J, Ondo W. Bruxism in Huntington's disease. Mov Disord 2000;15:171-3. [Crossref] [PubMed]
- Lavigne GJ, Khoury S, Abe S, et al. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil 2008;35:476-94. [Crossref] [PubMed]
- Jokubauskas L, Baltrušaitytė A. Relationship between obstructive sleep apnoea syndrome and sleep bruxism: a systematic review. J Oral Rehabil 2017;44:144-53. [Crossref] [PubMed]
- Bagattoni S, D'Alessandro G, Sadotti A, et al. Oro-dental features of Pallister-Killian syndrome: Evaluation of 21 European probands. Am J Med Genet A 2016;170:2357-64. [Crossref] [PubMed]
- Guo H, Wang T, Li X, et al. What sleep behaviors are associated with bruxism in children? A systematic review and meta-analysis. Sleep Breath 2017;21:1013-23. [Crossref] [PubMed]
- Castroflorio T, Bargellini A, Rossini G, et al. Risk factors related to sleep bruxism in children: A systematic literature review. Arch Oral Biol 2015;60:1618-24. [Crossref] [PubMed]
- Castroflorio T, Bargellini A, Rossini G, et al. Sleep bruxism in adolescents: a systematic literature review of related risk factors. Eur J Orthod 2017;39:61-8. [Crossref] [PubMed]
- Castroflorio T, Bargellini A, Rossini G, et al. Sleep bruxism and related risk factors in adults: A systematic literature review. Arch Oral Biol 2017;83:25-32. [Crossref] [PubMed]
- Firmani M, Reyes M, Becerra N, et al. Rev Chil Pediatr 2015;86:373-9. [Sleep bruxism in children and adolescents]. [Crossref] [PubMed]
- Grechi TH, Trawitzki LV, de Felício CM, et al. Bruxism in children with nasal obstruction. Int J Pediatr Otorhinolaryngol 2008;72:391-6. [Crossref] [PubMed]
- Chan J, Edman JC, Koltai PJ. Obstructive sleep apnea in children. Am Fam Physician 2004;69:1147-54. [PubMed]
- Serra-Negra JM, Paiva SM, Fulgêncio LB, et al. Environmental factors, sleep duration, and sleep bruxism in Brazilian schoolchildren: a case-control study. Sleep Med 2014;15:236-9. [Crossref] [PubMed]
- Oporto GH 5th, Bornhardt T, Iturriaga V, et al. Genetic polymorphisms in the serotonergic system are associated with circadian manifestations of bruxism. J Oral Rehabil 2016;43:805-12. [Crossref] [PubMed]
- Alfano CA, Bower JL, Meers JM. Polysomnography-Detected Bruxism in Children is Associated With Somatic Complaints But Not Anxiety. J Clin Sleep Med 2018;14:23-9. [Crossref] [PubMed]
- Ohlmann B, Bömicke W, Habibi Y, et al. Are there associations between sleep bruxism, chronic stress, and sleep quality? J Dent 2018;74:101-6. [Crossref] [PubMed]
- Soares-Silva L, Tavares-Silva C, Fonseca-Gonçalves A, et al. Presence of oral habits and their association with the trait of anxiety in pediatric patients with possible sleep bruxism. J Indian Soc Pedod Prev Dent 2019;37:245-50. [Crossref] [PubMed]
- Ramfjord SP. Bruxism, a clinical and electromyographic study. J Am Dent Assoc 1961;62:21-44. [Crossref] [PubMed]
- Lobbezoo F, Naeije M. Bruxism is mainly regulated centrally, not peripherally. J Oral Rehabil 2001;28:1085-91. [Crossref] [PubMed]
- Ribeiro-Lages MB, Martins ML, Magno MB, et al. Is there association between dental malocclusion and bruxism? A systematic review and meta-analysis. J Oral Rehabil 2020;47:1304-18. [Crossref] [PubMed]
- Apessos I, Andreadis D, Steiropoulos P, et al. Investigation of the relationship between sleep disorders and xerostomia. Clin Oral Investig 2020;24:1709-16. [Crossref] [PubMed]
- Winck M, Drummond M, Viana P, et al. Sleep bruxism associated with obstructive sleep apnoea syndrome - A pilot study using a new portable device. Rev Port Pneumol (2006) 2017;23:22-6. [PubMed]
- Han K, Wang C, Zhong Z, et al. Characterisation of the relationships between rhythmic masticatory muscle activities and limb movements in patients with sleep bruxism. J Oral Rehabil 2019;46:399-408. [Crossref] [PubMed]
- Zhang Y, Lu J, Wang Z, et al. Companion of oral movements with limb movements in patients with sleep bruxism: preliminary findings. Sleep Med 2017;36:156-64. [Crossref] [PubMed]
- Xu M, Zhong Z, Zou X, et al. Eye movements in relation to rhythmic masticatory muscle activities in patients with sleep bruxism. Sleep Med 2020;65:36-44. [Crossref] [PubMed]
- Wieckiewicz M, Bogunia-Kubik K, Mazur G, et al. Genetic basis of sleep bruxism and sleep apnea-response to a medical puzzle. Sci Rep 2020;10:7497. [Crossref] [PubMed]
- Abe Y, Suganuma T, Ishii M, et al. Association of genetic, psychological and behavioral factors with sleep bruxism in a Japanese population. J Sleep Res 2012;21:289-96. [Crossref] [PubMed]
- Hoashi Y, Okamoto S, Abe Y, et al. Generation of neural cells using iPSCs from sleep bruxism patients with 5-HT2A polymorphism. J Prosthodont Res 2017;61:242-50. [Crossref] [PubMed]
- Vieira AR, Scariot R, Gerber JT, et al. Bruxism Throughout the Lifespan and Variants in MMP2, MMP9 and COMT. J Pers Med 2020;10:44. [Crossref] [PubMed]
- Scariot R, Brunet L, Olsson B, et al. Single nucleotide polymorphisms in dopamine receptor D2 are associated with bruxism and its circadian phenotypes in children. Cranio 2022;40:152-9. [Crossref] [PubMed]
- Lobbezoo F, Visscher CM, Koutris M, et al. Bruxism in dentists' families. J Oral Rehabil 2018;45:657-8. [Crossref] [PubMed]
- Lobbezoo F, Visscher CM, Ahlberg J, et al. Bruxism and genetics: a review of the literature. J Oral Rehabil 2014;41:709-14. [Crossref] [PubMed]
- Fernandes G, van Selms MK, Gonçalves DA, et al. Factors associated with temporomandibular disorders pain in adolescents. J Oral Rehabil 2015;42:113-9. [Crossref] [PubMed]
- van Selms MK, Visscher CM, Naeije M, et al. Bruxism and associated factors among Dutch adolescents. Community Dent Oral Epidemiol 2013;41:353-63. [Crossref] [PubMed]
- Carra MC, Huynh N, Morton P, et al. Prevalence and risk factors of sleep bruxism and wake-time tooth clenching in a 7- to 17-yr-old population. Eur J Oral Sci 2011;119:386-94. [Crossref] [PubMed]
- Vinod KV, Reddy P, Pillai VM. Scalloped tongue: A rare finding in nocturnal bruxism. Natl Med J India 2017;30:296. [Crossref] [PubMed]
- Przystańska A, Jasielska A, Ziarko M, et al. Psychosocial Predictors of Bruxism. Biomed Res Int 2019;2019:2069716. [Crossref] [PubMed]
- Seraj B, Shahrabi M, Ghadimi S, et al. The Prevalence of Bruxism and Correlated Factors in Children Referred to Dental Schools of Tehran, Based on Parent's Report. Iran J Pediatr 2010;20:174-80. [PubMed]
- Nagamatsu-Sakaguchi C, Minakuchi H, Clark GT, et al. Relationship between the frequency of sleep bruxism and the prevalence of signs and symptoms of temporomandibular disorders in an adolescent population. Int J Prosthodont 2008;21:292-8. [PubMed]
- Ommerborn MA, Schneider C, Giraki M, et al. In vivo evaluation of noncarious cervical lesions in sleep bruxism subjects. J Prosthet Dent 2007;98:150-8. [Crossref] [PubMed]
- Cheifetz AT, Osganian SK, Allred EN, et al. Prevalence of bruxism and associated correlates in children as reported by parents. J Dent Child (Chic) 2005;72:67-73. [PubMed]
- Dias GM, Bonato LL, Guimarães JP, et al. A Study of the Association Between Sleep Bruxism, Low Quality of Sleep, and Degenerative Changes of the Temporomandibular Joint. J Craniofac Surg 2015;26:2347-50. [Crossref] [PubMed]
- Wetselaar P, Manfredini D, Ahlberg J, et al. Associations between tooth wear and dental sleep disorders: A narrative overview. J Oral Rehabil 2019;46:765-75. [Crossref] [PubMed]
- Demarco FF, Collares K, Coelho-de-Souza FH, et al. Anterior composite restorations: A systematic review on long-term survival and reasons for failure. Dent Mater 2015;31:1214-24. [Crossref] [PubMed]
- van Dijken JW, Pallesen U. Fracture frequency and longevity of fractured resin composite, polyacid-modified resin composite, and resin-modified glass ionomer cement class IV restorations: an up to 14 years of follow-up. Clin Oral Investig 2010;14:217-22. [Crossref] [PubMed]
- Rees JS, Somi S. A guide to the clinical management of attrition. Br Dent J 2018;224:319-23. [Crossref] [PubMed]
- Palinkas M, De Luca Canto G, Rodrigues LA, et al. Comparative Capabilities of Clinical Assessment, Diagnostic Criteria, and Polysomnography in Detecting Sleep Bruxism. J Clin Sleep Med 2015;11:1319-25. [Crossref] [PubMed]
- Lavigne GJ, Rompré PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res 1996;75:546-52. [Crossref] [PubMed]
- Avidan AY. Review of sleep medicine. 2018. ISBN 978-0-323-46216-7.
- Castroflorio T, Deregibus A, Bargellini A, et al. Detection of sleep bruxism: comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J Oral Rehabil 2014;41:163-9. [Crossref] [PubMed]
- McAuliffe P, Kim JH, Diamond D, et al. A sleep bruxism detection system based on sensors in a splint - pilot clinical data. J Oral Rehabil 2015;42:34-9. [Crossref] [PubMed]
- Restrepo C, Manfredini D, Castrillon E, et al. Diagnostic accuracy of the use of parental-reported sleep bruxism in a polysomnographic study in children. Int J Paediatr Dent 2017;27:318-25. [Crossref] [PubMed]
- Luiz de Barreto Aranha R, Nogueira Guimarães de Abreu MH, Serra-Negra JM, et al. Evidence-Based Support for Sleep Bruxism Treatment Other Than Oral Appliances Remains Insufficient. J Evid Based Dent Pract 2018;18:159-61. [Crossref] [PubMed]
- Manfredini D, Ahlberg J, Winocur E, et al. Management of sleep bruxism in adults: a qualitative systematic literature review. J Oral Rehabil 2015;42:862-74. [Crossref] [PubMed]
- Bellerive A, Montpetit A, El-Khatib H, et al. The effect of rapid palatal expansion on sleep bruxism in children. Sleep Breath 2015;19:1265-71. [Crossref] [PubMed]
- Ohmure H, Kanematsu-Hashimoto K, Nagayama K, et al. Evaluation of a Proton Pump Inhibitor for Sleep Bruxism: A Randomized Clinical Trial. J Dent Res 2016;95:1479-86. [Crossref] [PubMed]
- Macedo CR, Macedo EC, Torloni MR, et al. Pharmacotherapy for sleep bruxism. Cochrane Database Syst Rev 2014;CD005578. [PubMed]
- Goldstein G, DeSantis L, Goodacre C. Bruxism: Best Evidence Consensus Statement. J Prosthodont 2021;30:91-101. [Crossref] [PubMed]
- Bussadori SK, Motta LJ, Horliana ACRT, et al. The Current Trend in Management of Bruxism and Chronic Pain: An Overview of Systematic Reviews. J Pain Res 2020;13:2413-21. [Crossref] [PubMed]
- Tavares-Silva C, Holandino C, Homsani F, et al. Homeopathic medicine of Melissa officinalis combined or not with Phytolacca decandra in the treatment of possible sleep bruxism in children: A crossover randomized triple-blinded controlled clinical trial. Phytomedicine 2019;58:152869. [Crossref] [PubMed]
- Silva CT, Primo LG, Mangabeira A, et al. Homeopathic therapy for sleep bruxism in a child: Findings of a 2-year case report. J Indian Soc Pedod Prev Dent 2017;35:381-3. [Crossref] [PubMed]
- Dalewski B, Chruściel-Nogalska M, Frączak B. Occlusal splint versus modified nociceptive trigeminal inhibition splint in bruxism therapy: a randomized, controlled trial using surface electromyography. Aust Dent J 2015;60:445-54. [Crossref] [PubMed]
- Matsumoto H, Tsukiyama Y, Kuwatsuru R, et al. The effect of intermittent use of occlusal splint devices on sleep bruxism: a 4-week observation with a portable electromyographic recording device. J Oral Rehabil 2015;42:251-8. [Crossref] [PubMed]
- Macedo CR, Silva AB, Machado MA, et al. Occlusal splints for treating sleep bruxism (tooth grinding). Cochrane Database Syst Rev 2007;CD005514. [Crossref] [PubMed]
- Trindade M, Orestes-Cardoso S, de Siqueira TC. Interdisciplinary treatment of bruxism with an occlusal splint and cognitive behavioral therapy. Gen Dent 2015;63:e1-4. [PubMed]
- Rodrigues JA, Azevedo CB, Chami VO, et al. Sleep bruxism and oral health-related quality of life in children: A systematic review. Int J Paediatr Dent 2020;30:136-43. [Crossref] [PubMed]
- Egermark I, Carlsson GE, Magnusson T. A 20-year longitudinal study of subjective symptoms of temporomandibular disorders from childhood to adulthood. Acta Odontol Scand 2001;59:40-8. [Crossref] [PubMed]
- Ramos PFC, de Lima MDM, de Moura MS, et al. Breathing problems, being an only child and having parents with possible sleep bruxism are associated with probable sleep bruxism in preschoolers: a population-based study. Sleep Breath 2021;25:1677-84. [Crossref] [PubMed]
- Li Y, Yu F, Niu L, et al. Associations among Bruxism, Gastroesophageal Reflux Disease, and Tooth Wear. J Clin Med 2018;7:417. [Crossref] [PubMed]
- Cahlin BJ, Hedner J, Dahlström L. A randomised, open-label, crossover study of the dopamine agonist, pramipexole, in patients with sleep bruxism. J Sleep Res 2017;26:64-72. [Crossref] [PubMed]
- Wieckiewicz M, Paradowska-Stolarz A, Wieckiewicz W. Psychosocial aspects of bruxism: the most paramount factor influencing teeth grinding. Biomed Res Int 2014;2014:469187. [Crossref] [PubMed]
- de Ruiter MHT, Apperloo RC, Milstein DMJ, et al. Assessment of obstructive sleep apnoea treatment success or failure after maxillomandibular advancement. Int J Oral Maxillofac Surg 2017;46:1357-62. [Crossref] [PubMed]
- Kostrzewa-Janicka J, Jurkowski P, Zycinska K, et al. Sleep-Related Breathing Disorders and Bruxism. Adv Exp Med Biol 2015;873:9-14. [Crossref] [PubMed]
- Beddis H, Pemberton M, Davies S. Sleep bruxism: an overview for clinicians. Br Dent J 2018;225:497-501. [Crossref] [PubMed]
- Mengatto CM, Coelho-de-Souza FH, de Souza OB Junior. Sleep bruxism: challenges and restorative solutions. Clin Cosmet Investig Dent 2016;8:71-7. [Crossref] [PubMed]
- Bucci C, Amato M, Zingone F, et al. Prevalence of Sleep Bruxism in IBD Patients and Its Correlation to Other Dental Disorders and Quality of Life. Gastroenterol Res Pract 2018;2018:7274318. [Crossref] [PubMed]
- Fernandes G, Franco AL, Siqueira JT, et al. Sleep bruxism increases the risk for painful temporomandibular disorder, depression and non-specific physical symptoms. J Oral Rehabil 2012;39:538-44. [Crossref] [PubMed]
- Wetselaar P, Vermaire EJH, Lobbezoo F, et al. The prevalence of awake bruxism and sleep bruxism in the Dutch adult population. J Oral Rehabil 2019;46:617-23. [Crossref] [PubMed]
- Gomes MC, Neves ÉT, Perazzo MF, et al. Evaluation of the association of bruxism, psychosocial and sociodemographic factors in preschoolers. Braz Oral Res 2018;32:e009. [Crossref] [PubMed]
- Manfredini D, Lobbezoo F, Giancristofaro RA, et al. Association between proxy-reported sleep bruxism and quality of life aspects in Colombian children of different social layers. Clin Oral Investig 2017;21:1351-8. [Crossref] [PubMed]
Cite this article as: Thomas DC, Patel J, Kumar SS, Dakshinamoorthy J, Greenstein Y, Ravindran HK, Pitchumani PK. Sleep related bruxism—comprehensive review of the literature based on a rare case presentation. Front Oral Maxillofac Med 2024;6:3.