
The state of 3D printing of bioengineered customized maxillofacial bone scaffolds: a narrative review
Introduction
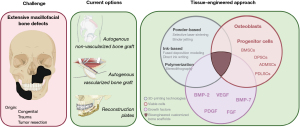
To regenerate large maxillofacial bone defects remains a great challenge in dental practice. Autologous bone, either vascularized or not, is still considered the gold standard for reestablishing microarchitecture, shape, and function when the bone gain is fundamental for the correction of congenital or acquired defects (due to trauma or excision of tumors). Titanium plates can also be applied for the correction of wide continuity defects (1,2). Within the maxillofacial area, the mandible is one of the most frequently affected bones. The reconstruction of segmental mandibular bone defects remains a major area of interest for surgeons and researchers, despite surgical and technical advances over the last decades (3-5).
The primary goal of maxillofacial reconstructive surgeries is to provide appropriate morphology and relationship between arches, restore hard and soft tissue, and guarantee sufficient height and width for insertion of endosseous titanium implants and further prosthetic rehabilitation. Its optimal features (osteogenicity, osteoinduction, and osteoconduction) support the autologous bone (collected either from intraoral or extraoral sites) as the most biologically favorable option for grafting. However, inherent limitations such as finite source and risk of infection of the donor site shall be considered alarming clinical challenges. Additionally, the rate of resorption of autologous bone is an incident and not desirable finding, compromising the long-term stability of the rehabilitations (2).
Rehabilitating completely and partially edentulous patients with dental implants has become increasingly popular, possibly due to the high long-term success rates of this modality. The reverse planning for the insertion of dental implants and prosthetic rehabilitation is achievable once the appropriate amount of bone was gained (6,7). In the past decades, the emergence of innovative technologies, such as tissue engineering (TE), may shift the paradigms of bone grafting and overcome the limitations of current techniques by providing customizable and individualized treatments (3,8,9). Bone tissue engineering (BTE) consists of an attractive minimally invasive approach to conventional forms of harvesting bone (4,10-12). Recently, virtual planning and 3D printing technologies have allowed the customization of scaffolds with precise dimensions, personalized structure, and complex morphologies for maxillofacial regeneration (4,5,13). Therefore, we aimed to assess what is known and what is to be known in order to safely and predictably apply these concepts for bone regeneration of large defects. We present this article in accordance with the Narrative Review reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-11/rc).
Methods
In this review, a detailed data-based search was performed following terms: maxillofacial bone regeneration, tissue engineering, 3D printing, stem cells, scaffolds, and growth factors (Table 1). The search was supplemented by checking references of relevant review articles. The search was performed on February 10th, 2022.
Table 1
| Items | Specification |
|---|---|
| Date of search | 02/10/2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Maxillofacial bone regeneration; tissue engineering; 3D printing; stem cells; scaffolds; growth factors |
| Timeframe | 02/03/1986–02/10/2022 |
| Inclusion and exclusion criteria | Included: human studies, prospective and retrospective clinical trials, in vivo studies, and systematic reviews in the English language |
| Selection process | The screening process was done by two independent authors (HRM and HH). Any disagreement during the screening was resolved by discussion with a third author (FPSG) |
BTE
Over the years, the knowledge evolved towards some optimal characteristics attributed to scaffolds for use in oral and maxillofacial surgery. These characteristics are biocompatibility, biodegradability, immunomodulation, adequate pores size, porosity, and interconnectivity. Also, mechanical resistance has been listed as an important factor in areas subjected to repeated/continuous loading (5).
Multiple manufacturing methods can be used: solvent casting, particle leaching, electrospinning, gas foaming, phase separation, etc. (10). However, the use of organic solvents may be limited due to the extended period of production, intense work, low replicability, pores with irregular shapes, and poor interconnectivity (10). The development and applicability of rapid prototyping technologies, such as 3D printing, have shown promising results in suppressing such limitations (3-5,10,14,15). 3D-printed scaffolds manufactured with bioactive functional materials and proper structure have been widely used for oral and maxillofacial regeneration (5,10,12,15,16).
The remarkable progress within BTE may turn reconstructive therapies into significantly less invasive procedures than gold-standard interventions, by suppressing the necessity of harvesting bone from intraoral or extraoral sites. Furthermore, patient morbidity, risk of infection, cost, and the duration of the intervention can be reduced. Also, the planning and reconstruction of bone defects with complex morphologies will be individualized rather than the standard interventions applied so far (17,18).
Biomaterial for scaffolds
Distinct synthetic polymers, such as polylactic acid (PLA), polyglycolic acid (PGA), copolymers of PLA and PGA [poly(lactic-co-glycolic acid) (PLGA)], and polycaprolactone (PCL) have been widely used for manufacturing bone scaffolds. Among a wide variety of materials (ceramics, bioactive glasses, polymers, and combinations), the chemical similarities with the natural bone mineral content allow synthetic calcium phosphates (CP) to be successfully used as a bone substitute (19,20).
Furthermore, scaffolds built with multiple compounds, such as combining PCL and β-tricalcium phosphate (β-TCP), aggregate the benefits of both polymers and ceramics and are successfully used for repairing mandibular defects in vivo (3,4). Additionally, synthetic biomaterials are not susceptible to immunological reactions, which may be compromising when natural scaffolds are chosen (20).
Stem cells
The ability of self-renewal (21) and multi-lineage differentiation of stem cells expanded their applicability in the era of regenerative medicine (22), presumably reaching the TE. Wehrle et al. (23) assessed the effects of loading a 3D-printed hydrogel scaffold (constructed with fibrin, gelatin, hyaluronic acid, glycerol, and hydroxyapatite) with mesenchymal stem cells (MSCs). Their results regarding mechanical stability, mineralization, and cell viability were promising.
The use of MSCs was also proven to be effective when loaded onto a 3D-printed β-TCP (24), which indicates that the beneficial properties of stem cells are maintained regardless of the material used for printing.
Other cell lines can be used for TE. Adipose-derived stem cells (ASCs) were largely investigated due to the easy obtention of considerable amounts of adipose tissue from the human body. Thus, this multipotent lineage has been widely used in TE, organ repair, and gene therapies (25). Studies loading 3D-printed scaffolds with ASCs should aim to assess whether their effectiveness is comparable or not to MSCs for BTE.
Bone morphogenetic proteins (BMPs)
The family of BMPs was firstly described by Urist in 1965 (26). There are approximately 30 proteins belonging to the family of BMPs, distributed in the bone matrix. Most of them belong to subfamilies within the transforming growth factor (TGF-β) superfamily (27).
BMP-2, BMP-6, and BMP-9 appear to be the most potent inductors of the differentiation of MSCs towards the osteoblastic lineage (28). BMPs activate the Smad pathway, which is a group of molecules that translocate and transmit the signals of receptors activated by BMPs to the cell nucleus (29). Albeit BMPs exert a beneficial influence on bone formation, some side effects may be present. Among them, the most common are edema, inflammation, and ectopic bone formation. Alarmingly, BMPs were associated with carcinogenesis (30).
Recombinant human BMP-2 (rhBMP-2) can be loaded onto absorbable collagen sponges (ACS). It was shown that this combination can support bone formation (31,32). Bone-forming cells migrate to the construct (rhBMP-2/ACS) and infiltrate within the ACS. The MSCs surrounding the construct increase in number, and the coupling of rhBMP-2 to MSCs receptors induce their differentiation in osteoblasts. As the sponge dissolves or is resorbed, cancellous bone and/or cartilage are formed concomitantly to angiogenesis. The process of bone formation is centripetal, from the outer to the inner region of the sponge and culminates with complete replacement by cancellous bone (31).
A clinical trial evaluated the bone formation capacity of an rhBMP-2/ACS construct in 80 post-extraction sockets (33). rhBMP-2 at the concentrations of 0.75 and 1.5 mg/mL were tested. Their results showed that the sockets treated with 1.5 mg/mL presented twice as much bone when compared with empty controls, maintaining crestal height and increasing thickness in 75%, 50%, and 25% of the original extraction socket. Furthermore, the histologic analysis evidenced the absence of differences between the new bone and the native bone.
The rhBMP-2 has been used for alveolar reconstruction, sinus lift, and socket healing. However, although their impact on pre-clinical and clinical trials is widely investigated, there is no consensus regarding the clinical efficacy of rhBMP-2 for regeneration of wide maxillofacial defects (34) (Figure 1).
3D printing
3D printing technologies, also known as additive manufacture or rapid prototyping, were firstly proposed in 1986, and since then have been rapidly widespread in dentistry due to their applications. It has revolutionized health systems through the manufacture of biomimetic implants, and customized prostheses. This process is interesting because of its great potential in producing scaffolds for use in BTE (15).
A scaffold can be 3D printed by means of rapid prototyping of clinical imaging, such as computed tomography (CT) or magnetic resonance imaging (MRI). The process, from image acquisition to the manufacture of the scaffold is as follows: a patient with a bone defect is submitted to an imaging exam and acquisition of DICOM (digital imaging and communication in medicine) files; the files are imported into the software for image processing; the images will be segmented and 3D models generated; the printing technique and the materials are selected; the actual printing will be performed for the obtention of the 3D scaffold; and, finally, subjected to post-processing (cleaning, thermal treatment, sterilization, and packing) (35). The thermal treatment and sterilization of the 3D-printed scaffolds are determined by the materials used for manufacturing. Hence, any inadequate post-processing steps can damage the structure of the scaffold and harm the final performance (Figure 2).
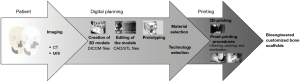
There are 3 main categories of 3D printing technologies that are most often used to produce customized bone scaffolds for maxillofacial regeneration. Powder-based, ink-based, and polymerization-based technologies. Within the powder-based category, the most used are selective laser sintering and binder jetting technologies; within the ink-based category, the most used are fused deposition modeling and direct ink writing; and within the polymerization category, the most used is stereolithography.
The manufacturing of individualized scaffolds based on medical imaging and computer modeling ought to be considered a promising technique for the regeneration of large defects in the maxillofacial region. The popularity of this technology in the dental field has increased, favoring low-cost and effective manufacturing while integrating the concepts of individualized care into dentistry.
Clinical applications of 3D printing for regeneration of bone defects
The clinical application of 3D-printed bone scaffolds seeded with MSCs has the capacity of revolutionizing the therapeutic options for oral and maxillofacial surgeons. The capacity of 3D printing in designing and manufacturing individualized and precise scaffolds is of great value and its beneficial application in the medical field is already proven (36-40). However, there are still some limitations needing to be overcome before large-scale clinical use. The lack of legal regulations, standardized procedures, randomized controlled clinical trials, and complete angiogenesis within the scaffolds need to be further assessed. In vitro, pre-clinical, and clinical settings need to be developed in order to accurately translate the application of 3D-printed scaffolds in the clinical scenario. Surgeons, researchers, industries, and regulatory agencies might build a workforce for overcoming the contemporary barriers and implementing this technology in daily practice, aiming to improve the quality of the treatment given to the patients.
Limitations
For a relevant clinical application, deep cellular penetration and angiogenesis are demanded. Vascularization is the main challenge in BTE (14,41,42). This initial angiogenesis is essential to the seeded cells viable. To achieve early angiogenesis, the implanted scaffold should contain pro-angiogenic factors (such as vascular endothelial growth factors secreted by osteoblasts) that will induce the formation and perfusion of new vessels from the surrounding connective tissue (14,41,42). Furthermore, the scaffold might allow the physical support for the vessels to permeate towards the center of the scaffold before extensive deposition of extracellular matrix (3). Also, with the objective of overcoming this barrier, two main options are proposed: (I) pre vascularization in vitro [seeding cells onto the scaffold; keeping the scaffold under static or dynamic (bioreactor) for speeding angiogenesis] and (II) in vivo pre-vascularization of the 3D-printed scaffolds (implanting the scaffold into axial vascular tissue prior to use, and posteriorly implantation in the actual recipient bed) (42).
Even 3D printing applied in the concept of BTE being promising for maxillofacial bone regeneration, regulatory conditions, large-scale manufacturing, safety, and costs remains relevant topics to be raised prior to considering the commerce and clinical application. Importantly, the available technique to the surgeon relies on scientific evidence and long-term follow-ups showing positive and negative points that should be considered when choosing the best clinical management for each patient.
Future perspectives
Novel bioactive synthetic materials in combination with strict protocols may contribute to the translation of stem-cell and growth factor therapies, providing cellular and molecular pathways to improve the healing process in the oral and maxillofacial region. Mimicking the 3D architecture and the functional biomechanics of the maxillofacial bone is a challenging proposition that demands strategic and personalized tissue replacement, which is not patient-specific so far.
Final considerations
Reconstruction and rehabilitation of the craniofacial region are great challenges to surgeons. As technology advances together with new therapeutic modalities, the ability to create bioactive tissues becomes more sophisticated. BTE is a well-established field of research from a pre-clinical point of view and actively develops products and devices standing for the principles of regenerative medicine (9-11,43).
As the future direction of the BTE becomes clearer and its applicability increases, is extremely important for researchers and clinicians working in this field to discuss and actively shift the pre-clinical studies towards clinical application. However, for BTW to reach daily clinical practice, the industry should be involved. Three main issues shall be considered during this process: economy, regulations, and manufacturing. The role of the industry is to efficiently make this technology marketable, scalable, manufacturable, and accessible for regulators, in order to directly improve the therapy for patients.
Finally, surgeons shall be alert to these advances, be capable of selecting appropriate techniques and materials (relying on the scientific evidence so far) and have the necessary ability for reconstructing oral and maxillofacial bone defects.
Acknowledgments
Funding: This work was supported in part by the MGH-Department of Oral and Maxillofacial Surgery Education Research Fund (Boston, MA, USA).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-11/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-22-11/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nkenke E, Neukam FW. Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol 2014;7:S203-17. [PubMed]
- Sethi A, Kaus T, Cawood JI, et al. Onlay bone grafts from iliac crest: a retrospective analysis. Int J Oral Maxillofac Surg 2020;49:264-71. [Crossref] [PubMed]
- Konopnicki S, Troulis MJ. Mandibular Tissue Engineering: Past, Present, Future. J Oral Maxillofac Surg 2015;73:S136-46. [Crossref] [PubMed]
- Tatara AM, Koons GL, Watson E, et al. Biomaterials-aided mandibular reconstruction using in vivo bioreactors. Proc Natl Acad Sci U S A 2019;116:6954-63. [Crossref] [PubMed]
- Shao H, Sun M, Zhang F, et al. Custom Repair of Mandibular Bone Defects with 3D Printed Bioceramic Scaffolds. J Dent Res 2018;97:68-76. [Crossref] [PubMed]
- Checchi V, Gasparro R, Pistilli R, et al. Clinical Classification of Bone Augmentation Procedure Failures in the Atrophic Anterior Maxillae: Esthetic Consequences and Treatment Options. Biomed Res Int 2019;2019:4386709. [Crossref] [PubMed]
- Nguyen TTH, Eo MY, Kuk TS, et al. Rehabilitation of atrophic jaw using iliac onlay bone graft combined with dental implants. Int J Implant Dent 2019;5:11. [Crossref] [PubMed]
- Aghaloo TL, Hadaya D. Basic Principles of Bioengineering and Regeneration. Oral Maxillofac Surg Clin North Am 2017;29:1-7. [Crossref] [PubMed]
- Melville JC, Mañón VA, Blackburn C, et al. Current Methods of Maxillofacial Tissue Engineering. Oral Maxillofac Surg Clin North Am 2019;31:579-91. [Crossref] [PubMed]
- Roseti L, Parisi V, Petretta M, et al. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater Sci Eng C Mater Biol Appl 2017;78:1246-62. [Crossref] [PubMed]
- Sparks DS, Saifzadeh S, Savi FM, et al. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat Protoc 2020;15:877-924. [Crossref] [PubMed]
- Mueller ML, Ottensmeyer MP, Thamm JR, et al. Increased Osteogenic Activity of Dynamic Cultured Composite Bone Scaffolds: Characterization and In Vitro Study. J Oral Maxillofac Surg 2022;80:303-12. [Crossref] [PubMed]
- Largo RD, Garvey PB. Updates in Head and Neck Reconstruction. Plast Reconstr Surg 2018;141:271e-85e. [Crossref] [PubMed]
- Bhumiratana S, Bernhard JC, Alfi DM, et al. Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med 2016;8:343ra83. [Crossref] [PubMed]
- Maroulakos M, Kamperos G, Tayebi L, et al. Applications of 3D printing on craniofacial bone repair: A systematic review. J Dent 2019;80:1-14. [Crossref] [PubMed]
- Guastaldi FPS, Takusagawa T, Monteiro JLGC, et al. 3D Printing for Oral and Maxillofacial Regeneration. In: Guastaldi FP, Mahadik B. editors. Bone Tissue Engineering: Bench to Bedside Using 3D Printing. Cham: Springer Publishing, 2022.
- Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater 2017;2:224-47. [Crossref] [PubMed]
- Tellisi N, Ashammakhi NA, Billi F, et al. Three Dimensional Printed Bone Implants in the Clinic. J Craniofac Surg 2018;29:2363-7. [Crossref] [PubMed]
- Dorozhkin SV. Calcium orthophosphates: occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011;1:121-64. [Crossref] [PubMed]
- Williams DF. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front Bioeng Biotechnol 2019;7:127. [Crossref] [PubMed]
- Fu X, Liu G, Halim A, et al. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019;8:784. [Crossref] [PubMed]
- Trohatou O, Roubelakis MG. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell Reprogram 2017;19:217-24. [Crossref] [PubMed]
- Wehrle M, Koch F, Zimmermann S, et al. Examination of hydrogels and mesenchymal stem cell sources for bioprinting of artificial osteogenic tissues. Cell Mol Bioeng 2019;12:583-97. [Crossref]
- Nair MA, Shaik KV, Kokkiligadda A, et al. Tissue-engineered Maxillofacial Skeletal Defect Reconstruction by 3D Printed Beta-tricalcium phosphate Scaffold Tethered with Growth Factors and Fibrin Glue Implanted Autologous Bone Marrow-Derived Mesenchymal Stem Cells. J Med Life 2020;13:418-25. [Crossref] [PubMed]
- Moreno M, Amaral MH, Lobo JM, et al. Scaffolds for Bone Regeneration: State of the Art. Curr Pharm Des 2016;22:2726-36. [Crossref] [PubMed]
- Urist MR. Bone: formation by autoinduction. Science 1965;150:893-9. [Crossref] [PubMed]
- Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int 2000;57:2207-14. [Crossref] [PubMed]
- Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am 2003;85:1544-52. [Crossref] [PubMed]
- Deschaseaux F, Sensébé L, Heymann D. Mechanisms of bone repair and regeneration. Trends Mol Med 2009;15:417-29. [Crossref] [PubMed]
- James AW, LaChaud G, Shen J, et al. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev 2016;22:284-97. [Crossref] [PubMed]
- Ben Amara H, Lee JW, Kim JJ, et al. Influence of rhBMP-2 on Guided Bone Regeneration for Placement and Functional Loading of Dental Implants: A Radiographic and Histologic Study in Dogs. Int J Oral Maxillofac Implants 2017;32:e265-76. [Crossref] [PubMed]
- Li F, Yu F, Liao X, et al. Efficacy of Recombinant Human BMP2 and PDGF-BB in Orofacial Bone Regeneration: A Systematic Review and Meta-analysis. Sci Rep 2019;9:8073. [Crossref] [PubMed]
- Fiorellini JP, Howell TH, Cochran D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol 2005;76:605-13. [Crossref] [PubMed]
- Carreira AC, Lojudice FH, Halcsik E, et al. Bone morphogenetic proteins: facts, challenges, and future perspectives. J Dent Res 2014;93:335-45. [Crossref] [PubMed]
- Wong ME, Kau CH, Melville JC, et al. Bone Reconstruction Planning Using Computer Technology for Surgical Management of Severe Maxillomandibular Atrophy. Oral Maxillofac Surg Clin North Am 2019;31:457-72. [Crossref] [PubMed]
- Rachmiel A, Shilo D, Blanc O, et al. Reconstruction of complex mandibular defects using integrated dental custom-made titanium implants. Br J Oral Maxillofac Surg 2017;55:425-7. [Crossref] [PubMed]
- Wiltfang J, Rohnen M, Egberts JH, et al. Man as a Living Bioreactor: Prefabrication of a Custom Vascularized Bone Graft in the Gastrocolic Omentum. Tissue Eng Part C Methods 2016;22:740-6. [Crossref] [PubMed]
- Ahn G, Lee JS, Yun WS, et al. Cleft Alveolus Reconstruction Using a Three-Dimensional Printed Bioresorbable Scaffold With Human Bone Marrow Cells. J Craniofac Surg 2018;29:1880-3. [Crossref] [PubMed]
- Naujokat H, Açil Y, Gülses A, et al. Man as a living bioreactor: Long-term histological aspects of a mandibular replacement engineered in the patient's own body. Int J Oral Maxillofac Surg 2018;47:1481-7. [Crossref] [PubMed]
- Mueller ML, Thamm JR, Troulis MJ, et al. Clinical application of three-dimensional printing and tissue engineering for maxillofacial reconstruction. A Review of Reported Cases. JSM Oro Facial Surg 2020;4:1013.
- Kasper FK, Melville J, Shum J, et al. Tissue Engineered Prevascularized Bone and Soft Tissue Flaps. Oral Maxillofac Surg Clin North Am 2017;29:63-73. [Crossref] [PubMed]
- Tian T, Zhang T, Lin Y, et al. Vascularization in Craniofacial Bone Tissue Engineering. J Dent Res 2018;97:969-76. [Crossref] [PubMed]
- Guastaldi AC, Barbosa GF, Carvalho J, et al. Bioimpressão 3D no contexto da Indústria 4.0 aplicada à saúde. São Carlos: EdUFSCar, 2021.
Cite this article as: Matheus HR, Hadad H, Guastaldi FPS. The state of 3D printing of bioengineered customized maxillofacial bone scaffolds: a narrative review. Front Oral Maxillofac Med 2024;6:5.

