
Surgery first: current state of the art orthognathic surgery and its potential as a primary treatment modality in obstructive sleep apnea with concurrent dentofacial deformities
Introduction
Orthognathic surgery is an effective treatment modality in patients with either dentofacial deformities (DFD) or obstructive sleep apnea (OSA). The concept of orthognathic “surgery first” has been discussed in the literature and employed in practice for quite some time for the management of DFD. Due to advancements in three-dimensional planning, it is being utilized more in patients with DFD resulting in an overall decrease in treatment time (1-3). As orthognathic surgery is a treatment modality for multiple conditions, it should be explored more in the subset of patients with concurrent DFD and OSA. The purpose of this paper is to highlight the rationale to employ orthognathic surgery as a primary treatment modality in patients with concurrent OSA and DFD. A brief review of OSA and two cases of DFD in which the surgery first approach (SFA) was applied will be discussed. We present the following article in accordance with the AGREE reporting checklist (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-61/rc).
Definitions
DFD are conditions impacting the normal proportions of the facial skeleton and occlusion. Maxillomandibular abnormalities not only affect functional aspects, it impacts psychological and esthetic factors as well. OSA belongs to a group of disorders ranging from habitual snoring to moderate or severe obstructive sleep apnea syndrome (OSAS) which is characterized by the cyclic cessation of breathing and or hypopnea during sleep (4). Key features of OSAS are daytime hyper-somnolence and polysomnography proven obstructive apneas (5).
SFA is the orthognathic surgery performed prior to orthodontic preparation (6).
Epidemiology
DFD affect close to 20% of the population in various degrees of occlusion and function. OSA is estimated to impact between 27% and 43% of women and men respectively (7). OSA patients are usually older and have multiple comorbidities compared to patients undergoing orthognathic surgery for DFD (8). In the United States, DFD contributing to malocclusion which require surgical correction is estimated to be 2.7% (9).
Etiology
Malocclusion and associated skeletal abnormalities of the face are due to numerous factors including genetics, prenatal problems, obesity, systemic conditions that occur during growth, trauma, and environmental influences (9). The etiology of OSA relates to inspiratory airway pressures that overcome muscular forces opposing airway collapse. During sleep, relaxation of the upper airway tongue and pharyngeal muscles results in airway narrowing (4). In order to maintain a patent airway during inspiration, the genioglossus muscle is activated at the same time as the inspiratory muscles. In OSA, activation of the genioglossus is reduced which (10) leads to apneic and hypopneic events and in turn results in fragmented sleep (4). Risk factors associated with OSA include anatomically narrowed airway, high blood pressure, asthma, obesity, smoking and diabetes.
Pathology
The pathophysiology of OSA relates to sporadic hypoxia and recurring nighttime arousals which disrupt the sleep cycle. This causes increased sympathetic activation and oxidative stress which contributes to development of endothelial dysfunction, increased platelet aggregation, coronary artery disease, heart failure, metabolic syndrome and cerebrovascular accidents (11). Hypoxia can cause pulmonary vascular remodeling resulting in pulmonary hypertension and right ventricular hypertrophy (12). Of note, studies show a higher mortality rate among OSA patients with concomitant cardiovascular disease (13).
Clinical presentation
OSA patients may or may not show good facial proportions. Computerized tomography (CT) scans or lateral cephalograms may demonstrate decreased upper airway space. However, most imaging is not taken in the supine position and therefore not the best indicator of decreased airway space. The mandibular plane to hyoid distance is a predictor of OSA. The upper airway can be approximated by measuring the distance from the mandibular plane to the hyoid. It’s thought that lower-set hyoid bones correlate to soft tissue laxity and airway collapse (14).
In general, OSA patients have reduced slow wave sleep leading to symptoms of daytime somnolence, morning headaches, poor concentration, memory loss and depression which in turn can cause marital discord and increased risk of car accidents (4).
Diagnosis
An abnormal apnea-hypopnea index (AHI) is necessary for disease classification and is diagnosed by a sleep physician. OSA severity is based on the number of apnea and hypopnea’s occurring per hour of sleep. OSA is classified as mild if the AHI is 5–15, moderate 15–30 and severe if greater than 30 (15). Severe OSA occurs in 10–20% of patients with a BMI greater than 35 (16). Apnea and hypopnea’s observed on polysomnography is the current gold standard for diagnosis of OSA (17). Airway collapse can be visualized on sleep endoscopy (18).
The literature mentions several craniofacial traits that are common in patients with OSAS. These include “bimaxillary retrusion, mandibular deficiency, short cranial base, reduced cranial base angle and mandibular length, increased lower anterior facial height, inferiorly positioned hyoid and an enlarged soft palate” (19). Mandibular deficiency in relation to the maxilla is the most common skeletal abnormality predisposing to OSA (20).
Management of OSA
Normalization of the AHI is a key treatment objective, however studies indicate permanent neuroanatomic effects of OSA in some patients (21). As well, it is worth noting that up to 22% of people have residual hypersomnia after normalization of the AHI with positive airway pressure therapy (22,23).
Continuous positive airway pressure (CPAP) is considered the “gold” standard for OSA treatment. It is effective when used properly, however, effectiveness is low due to poor patient adherence. Surgery for OSA does not rely on long-term patient adherence and long-term results have been shown to be successful (4). Patients likely to benefit from maxillomandibular advancement (MMA) are those with true anteroposterior collapse of the airway and decreased airway space. MMA should be carefully planned in order to optimize aesthetics, prevent excessive incisor show and excessive protrusion of the lower facial third. These considerations are even more important in OSA patients with concurrent DFD.
Airway narrowing patterns have been classified in the following manner: type I collapse involves narrowing of the retropalatal region; type II includes narrowing or collapse of both the retropalatal and retrolingual areas; and type III collapse occurs only in the retrolingual area. MMA improves patency of both retropalatal and retrolingual spaces (24).
In regards to OSA, surgical candidates include adult’s intolerant to CPAP and oral appliances or those whose anatomical features impair proper fit, as well as adults with anatomical narrowing of the pharynx (e.g., macroglossia, retrognathia) and adults who refuse to use a CPAP device (25).
Alternatives to MMA primarily involve soft tissue rather than bony surgery and include uvulopalatopharyngoplasty, hyoid suspension, epiglottoplasty, and implantable neurostimulation devices (26).
3D imaging techniques and virtual surgical simulations have greatly improved surgical predictability and outcomes. Cone beam computerized tomography (CBCT) imaging (27,28) and 3D intra-oral scans (29,30) have allowed for easy image capture, 3D segmentation and also visualization. In addition, Orthodontists and Oral Maxillofacial Surgeons may now communicate on web-based platforms for better communication of the surgical plan (31) and planning the final occlusion (32). Finally, 3D manufacturing of the surgical wafers and splints transfer the necessary information from the plan to the surgical table (33).
Case 1
A 46-year-old man presented at the Department of Orthodontics, University of Alabama at Birmingham for consultation. After extensive exploration and summarizing findings, patient received a diagnosis of Skeletal Class II malocclusion and his medical condition included OSA. Clinical evaluation exhibits lip competence with minimal gingival display at smiling. The patient had a retruded chin with no significant facial asymmetry giving the appearance of a concave profile.
Intraorally, his mandibular third molars were present. He had high palatal vault, rounded maxillary and mandibular arches, maxillary dental spacing and mild lower crowding (Figure 1). Only permanent teeth are present, mandibular left first molar and maxillary third molars are missing (Figure 2). His upper and lower dental midline was not coincident due to the mandibular dental crowding. A SFA was planned to conclude with orthodontic treatment (Figure 3 and Table 1).
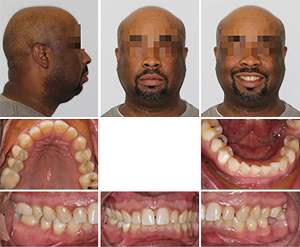
Table 1
| Group/measurement | Value | Norm |
|---|---|---|
| Skeletal | ||
| Sella-nasion-A point (SNA) (°) | 91.5 | 82.0 |
| Sella-nasion-B point (SNB) (°) | 78.6 | 80.9 |
| Point A-nasion-B point (ANB) (°) | 12.9 | 1.6 |
| Wits appraisal (mm) | 6.5 | −1.0 |
| Skeletal vertical | ||
| Mandibular plane-sella-nasion (MP-SN) (°) | 35.5 | 33.0 |
| Frankfort horizontal plane-mandibular plane angle (MP-FH) (°) | 26.1 | 22.9 |
| Dental | ||
| Overjet (mm) | 5.6 | 2.5 |
| Overbite (mm) | 2.0 | 2.5 |
| Interincisal angle (U1-L1) (°) | 113.8 | 130.0 |
| Upper incisors-sella nasion (U1-SN) (°) | 105.1 | 103.1 |
| Upper incisors-nasion-A point A (U1-NA) (mm) | 0.5 | 4.3 |
| Lower incisors-nasion-point B (L1-NB) (mm) | 13.6 | 4.0 |
| Lower incisors-mandibular plane (L1-MP) (°) | 105.4 | 95.0 |
| Soft tissue | ||
| Lower lip to E-plane (mm) | 9.1 | −2.0 |
| Upper lip to E-plane (mm) | 4.7 | −8.0 |
After orthognathic surgery, 0.018×0.018 NiTi archwires were placed on both arches along with Class III elastics (Figure 4).
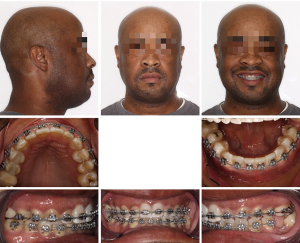
Subsequently, 0.016×0.022 NiTi wires were changed and the case was finished on 0.014×0.018 NiTi archwires. The overall treatment time was 11 months from start to finish (Figures 5,6). Retention protocol: upper and lower Essix retainers.
Case 2
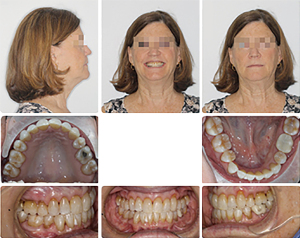
This 63-year-old female presented with OSA. Extensive examination reveals a concave profile, Class III molar relation, moderate crowding of lower anterior teeth, dental midlines matching and ovoid maxillary and mandibular arches (Figures 7 and 8). The extraoral examination revealed her upper and lower lips to be retrusive according to the E-plane. Lower lip is positioned −7.7 mm whereas her upper lip is at −9.3 mm relative to the Rickett’s E-plane (Figure 9 and Table 2).
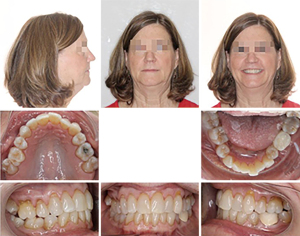
Table 2
| Group/measurement | Value | Norm |
|---|---|---|
| Skeletal | ||
| Sella-nasion-A point (SNA) (°) | 81.5 | 82.0 |
| Sella-nasion-B point (SNB) (°) | 81.2 | 80.9 |
| Point A-nasion- B point (ANB) (°) | 0.3 | 1.6 |
| Wits sppraisal (mm) | −1.9 | −1.0 |
| Skeletal vertical | ||
| Mandibular plane-sella-nasion (MP-SN) (°) | 30.9 | 33.0 |
| Frankfort horizontal plane-mandibular plane angle (MP-FH) (°) | 27.1 | 23.9 |
| Dental | ||
| Overjet (mm) | 2.8 | 2.5 |
| Overbite (mm) | 1.7 | 2.5 |
| Interincisal angle (U1-L1) (°) | 143.9 | 130.0 |
| Upper incisors-sella nasion (U1-SN) (°) | 100.4 | 102.8 |
| Upper incisors-nasion-A point A (U1-NA) (mm) | 4.8 | 4.3 |
| Lower incisors-nasion-point B (L1-NB) (mm) | 2.5 | 4.0 |
| Lower incisors-mandibular plane (L1-MP) (°) | 82.4 | 95.0 |
| Soft tissue | ||
| Lower lip to E-plane (mm) | −7.7 | −2.0 |
| Upper lip to E-plane (mm) | −9.3 | −6.0 |
Bimaxillary surgery with surgery-first approach was performed and immediate movement of the teeth with orthodontic treatment started after surgery considering the regional acceleratory phenomenon (34). Overall treatment last for approximately 11 months.
After orthognathic surgery, 0.014 NiTi wires were placed on both arches along with Class II elastics (Figures 10-12).
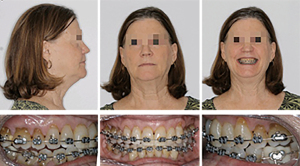
Subsequently, 0.016×0.022 NiTi wires were changed and the case was finished on 0.018×0.018 stainless steel archwires. The overall treatment time was 11 months from start to finish (Figures 13,14). Retention protocol: upper Essix retainer and lower fixed retainer.
The cases showed in this paper, where patients with OSA, provide examples of well-achieved outcomes from the aesthetics, function and satisfaction standpoint. The patients benefitted in a short time after surgery and orthodontic treatment without compromising the results. Proving that the surgical first approach can be an effective treatment modality for patients with OSA.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The literature shows all malocclusions and DFD are amenable to the SFA. Advantages of this treatment modality include immediate resolution of the dentofacial deformity, easier decompensation of the malocclusion after surgery and significant reduction in therapy time. A 2014 systematic review reported treatment duration was approximately less than a year (35).
Orthognathic surgery for DFD and OSA has undergone significant philosophical change. Research is focusing on the patient phenotype most likely to benefit from orthognathic surgery versus other surgical options. Customized treatment plans based on the dentofacial deformity and locations of airway obstruction can target the etiology more effectively. Proper manipulation of skeletal components can effectively improve quality of life and sleep for patients (36).
As mentioned, elevations in AHI correlate with elevated risk of cardiovascular sequelae, symptoms, and neurocognitive effects (37). Studies have shown that improvement in AHI decreased all of the above (38,39). Reduction in OSA through orthognathic surgery will likely decrease these adverse events highlighting why definitive management of OSA through a SFA should be considered as a primary modality.
For patients with combined skeletal deformities and OSA that requires MMA, it is important to consider total treatment length. For DFD, the overall orthodontic treatment period for the conventional approach ranged from 22 to 36 months, compared to SFA which ranged from 10 to 14 months (1-3), through the regional acceleratory phenomenon.
Although the concept of a SFA was introduced decades ago, imaging was limited to two-dimensions. With advancements of three-dimensional imaging, prediction of surgical outcome is more accurate, especially in OSA patients (40). Uribe et al. showed favorable esthetic and occlusal outcomes after surgical correction of facial asymmetry with the SFA (41).
Conclusions
Based on our experience with decreased total treatment time for patients undergoing combined orthodontic and orthognathic surgery for correction of DFD, the authors believe patients who phenotypically have a decrease in anterior-posterior facial dimensions resulting in a combination of dentofacial facial deformity, malocclusion and OSA should be considered for SFA management. This would be accomplished best through close collaboration between the orthodontist, sleep medicine specialist and OMFS to identify and plan treatment for this subset of patients.
From the literature and included case reports, it can be deduced that patients with concurrent DFD and OSA due to skeletal deficiency may be managed more effectively (i.e., length of treatment and overall results) via a SFA. This highlights a need in the literature to evaluate overall outcomes in patients with concurrent DFD and OSA who are treated via a SFA. Important metrics would include total treatment time, improvement in AHI and morbidity, as well as a subjective assessment of QOL changes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AGREE reporting checklist. Available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-61/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-61/coif). CHK serves as an unpaid editorial board member of Frontiers of Oral and Maxillofacial Medicine from July 2020 to June 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jeong WS, Choi JW, Kim DY, et al. Can a surgery-first orthognathic approach reduce the total treatment time? Int J Oral Maxillofac Surg 2017;46:473-82. [Crossref] [PubMed]
- Slavnic S, Marcusson A. Duration of orthodontic treatment in conjunction with orthognathic surgery. Swed Dent J 2010;34:159-66. [PubMed]
- Uribe F, Adabi S, Janakiraman N, et al. Treatment duration and factors associated with the surgery-first approach: a two-center study. Prog Orthod 2015;16:29. [Crossref] [PubMed]
- Rotenberg BW, Vicini C, Pang EB, et al. Reconsidering first-line treatment for obstructive sleep apnea: a systematic review of the literature. J Otolaryngol Head Neck Surg 2016;45:23. [Crossref] [PubMed]
- Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med 1976;27:465-84. [Crossref] [PubMed]
- Nagasaka H, Sugawara J, Kawamura H, et al. "Surgery first" skeletal Class III correction using the Skeletal Anchorage System. J Clin Orthod 2009;43:97-105. [PubMed]
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310-8. [Crossref] [PubMed]
- Passeri LA, Choi JG, Kaban LB, et al. Morbidity and Mortality Rates After Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea. J Oral Maxillofac Surg 2016;74:2033-43. [Crossref] [PubMed]
- Hupp JR, Ellis E, Tucker MR. Contemporary oral and maxillofacial surgery. 2014.
- Fassbender P, Herbstreit F, Eikermann M, et al. Obstructive Sleep Apnea-a Perioperative Risk Factor. Dtsch Arztebl Int 2016;113:463-9. [PubMed]
- Ayas NT, Taylor CM, Laher I. Cardiovascular consequences of obstructive sleep apnea. Curr Opin Cardiol 2016;31:599-605. [Crossref] [PubMed]
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009;51:363-70. [Crossref] [PubMed]
- Giles TL, Lasserson TJ, Smith BJ, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006;CD001106. [PubMed]
- Riha RL, Brander P, Vennelle M, et al. A cephalometric comparison of patients with the sleep apnea/hypopnea syndrome and their siblings. Sleep 2005;28:315-20. [PubMed]
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89. [Crossref] [PubMed]
- Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology 2014;121:707-18. [Crossref] [PubMed]
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597-619. [Crossref] [PubMed]
- Soose RJ. Novel Surgical Approaches for the Treatment of Obstructive Sleep Apnea. Sleep Med Clin 2016;11:189-202. [Crossref] [PubMed]
- Lam B, Ip MS, Tench E, et al. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax 2005;60:504-10. [Crossref] [PubMed]
- Miles PG, Vig PS, Weyant RJ, et al. Craniofacial structure and obstructive sleep apnea syndrome--a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop 1996;109:163-72. [Crossref] [PubMed]
- Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med 2006;2:461-71. [Crossref] [PubMed]
- Kordbacheh Changi K, Finkelstein J, Papapanou PN. Peri-implantitis prevalence, incidence rate, and risk factors: A study of electronic health records at a U.S. dental school. Clin Oral Implants Res 2019;30:306-14. [Crossref] [PubMed]
- Weaver TE, Chasens ER, Arora S. Modafinil improves functional outcomes in patients with residual excessive sleepiness associated with CPAP treatment. J Clin Sleep Med 2009;5:499-505. [Crossref] [PubMed]
- Sher AE. Upper airway surgery for obstructive sleep apnea. Sleep Med Rev 2002;6:195-212. [Crossref] [PubMed]
- Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010;33:1408-13. [Crossref] [PubMed]
- Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014;370:139-49. [Crossref] [PubMed]
- Wang J, Veiszenbacher E, Waite PD, et al. Comprehensive treatment approach for bilateral idiopathic condylar resorption and anterior open bite with customized lingual braces and total joint prostheses. Am J Orthod Dentofacial Orthop 2019;156:125-36. [Crossref] [PubMed]
- Wong ME, Kau CH, Melville JC, et al. Bone Reconstruction Planning Using Computer Technology for Surgical Management of Severe Maxillomandibular Atrophy. Oral Maxillofac Surg Clin North Am 2019;31:457-72. [Crossref] [PubMed]
- Torassian G, Kau CH, English JD, et al. Digital models vs plaster models using alginate and alginate substitute materials. Angle Orthod 2010;80:474-81. [Crossref] [PubMed]
- Kau CH, Littlefield J, Rainy N, et al. Evaluation of CBCT digital models and traditional models using the Little's Index. Angle Orthod 2010;80:435-9. [Crossref] [PubMed]
- Kyteas PG, McKenzie WS, Waite PD, et al. Comprehensive treatment approach for condylar hyperplasia and mandibular crowding with custom lingual braces and 2-jaw surgery. Am J Orthod Dentofacial Orthop 2017;151:174-85. [Crossref] [PubMed]
- Kau CH, Almakky O, Louis PJ. Team approach in the management of revision surgery to correct bilateral temporomandibular joint replacements. J Orthod 2020;47:156-62. [Crossref] [PubMed]
- Oueis R, Waite PD, Wang J, et al. Orthodontic-Orthognathic Management of a patient with skeletal class II with bimaxillary protrusion, complicated by vertical maxillary excess: A multi-faceted case report of difficult treatment management issues. Int Orthod 2020;18:178-90. [Crossref] [PubMed]
- Delanian S, Chatel C, Porcher R, et al. Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherol-clodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys 2011;80:832-9. [Crossref] [PubMed]
- Huang CS, Hsu SS, Chen YR. Systematic review of the surgery-first approach in orthognathic surgery. Biomed J 2014;37:184-90. [Crossref] [PubMed]
- Hsieh YJ, Liao YF. Effects of maxillomandibular advancement on the upper airway and surrounding structures in patients with obstructive sleep apnoea: a systematic review. Br J Oral Maxillofac Surg 2013;51:834-40. [Crossref] [PubMed]
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19-25. [Crossref] [PubMed]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [Crossref] [PubMed]
- Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med 2002;166:159-65. [Crossref] [PubMed]
- Jung J, Moon SH, Kwon YD. Current status of surgery-first approach (part III): the use of 3D technology and the implication in obstructive sleep apnea. Maxillofac Plast Reconstr Surg 2020;42:1. [Crossref] [PubMed]
- Uribe F, Janakiraman N, Shafer D, et al. Three-dimensional cone-beam computed tomography-based virtual treatment planning and fabrication of a surgical splint for asymmetric patients: surgery first approach. Am J Orthod Dentofacial Orthop 2013;144:748-58. [Crossref] [PubMed]
Cite this article as: Pearl C, Chubb DWR, Marchena JM, Waite P, Buendia AM, Kau CH. Surgery first: current state of the art orthognathic surgery and its potential as a primary treatment modality in obstructive sleep apnea with concurrent dentofacial deformities. Front Oral Maxillofac Med 2022;4:29.










