
Inferior alveolar and lingual nerve injuries: an overview of diagnosis and management
Introduction
Nerve repair for an iatrogenic injury is evaluated separately for motor and sensory nerves, the former requiring urgent exploration and surgical intervention. On the other hand, injuries to sensory nerves are less time sensitive (1).
In 1951, Sunderland have proposed a classification for nerve injuries similar to the one of Seddon’s. Sunderland’s first-degree injury corresponds with Seddon’s neuropraxia and is the mildest form of injury. Second-, third- and fourth-degree injuries are parallel to axonotmesis and the fifth-degree, which is the complete transection of the nerve, is compatible with neurotmesis (2). The prognosis for spontaneous recovery is inversely proportional to the classification, meaning that excellent, good, fair, poor or no recovery may be expected without any surgical repair or approximation in injuries from first to fifth degrees, respectively (3).
Oral and maxillofacial surgical procedures are susceptible to cause injury to terminal branches of the trigeminal nerve. Although a vast majority of these injuries do not require further intervention and undergo spontaneous neurosensory recovery, they may sometimes result in serious functional deficits (4). Such cases have debilitating outcomes on orofacial function, and consequently a significant impact on the quality of life (1).
The mandibular division of the trigeminal nerve is more susceptible to injury than ophthalmic and maxillary nerves (5). Inferior alveolar nerve (IAN) branch of the trigeminal nerve is the most commonly injured branch, followed by the lingual nerve (LN) (5,6). These nerves are subjected to neurosensorial disturbance (NSD) during third molar surgery, followed by sagittal split ramus osteotomy (SSRO), endodontic therapy and dental implant placement (1). Pre-prosthetic surgery, local anesthetic injections, various types of orthognathic surgery, ablative tumor surgery involving mandibular resections, osteoradionecrosis, osteomyelitis or maxillofacial trauma are among other potential etiologic factors (1,7). The risk of mandibular nerve injury during different procedures ranges between 0.54–39% (5).
The authors refrain from listing clear surgical indications or contraindications for nerve repair but rather make decisions based on both clinical findings and the patients’ subjective assessment on their clinical status. Accordingly, surgery is indicated for Sunderland Grade IV and V injuries either witnessed, or unwitnessed but associated with severe or complete sensory deficit through neurosensory tests (NSTs) at 2 to 6 months or by magnetic resonance neurography (MRN) any time (2). Yet, if a patient is not bothered by their clinical status, ultimately surgery is contraindicated, as the loss of sensation would likely not get worse in the future.
Data regarding nerve injuries may not always be reliable since most are based upon personal experience and in a retrospective nature (1). It is also challenging to draw proper conclusions from studies on nerve injuries due to the differences in outcome criteria and assessment methods (8). Still, an accurate knowledge of anatomy should be combined with both clinical and radiological data to successfully avoid any nerve-related complications (6). This narrative review will aim to answer how inferior alveolar and LN injuries occur and are managed, and what the future trends are in nerve repairs, through evaluation of the current literature.
Materials and methods
A comprehensive literature research was conducted using PubMed database to identify studies published on inferior alveolar or LN injuries and their repair. The research comprised the following keywords and Medical Subject Headings (MeSH) terms: [“lingual nerve” OR “inferior alveolar nerve”] AND [“nerve repair” OR “nerve injury” OR “management” OR “treatment”]. Among the resulting articles, abstracts were reviewed for their relevance and then were retrieved for full-text analysis. Animal studies and single case reports, as well as articles not written in English or articles unavailable for their final full-text assessment were excluded. In order to keep this review as up-to-date as possible, systematic reviews and meta-analyses published within the last 5 years (between years 2015–2020) were prioritized.
Discussion
Functional assessment methods
IAN and LN injuries occur mainly due to iatrogenic causes and thus these may, at times, be difficult and troublesome to explain to patients. A thorough clinical evaluation and accurate assessment is crucial, and physicians must have a formal documentation of the complication both for better outcomes in treatment and also for legal conflicts that might arise thereafter. Before any method of assessment, it is important to have an accurate record of the area where a patient experiences the neurosensory deficit with a photograph to be able to use it in future comparisons. The patient’s own subjective assessment is considered the most sensitive indicator since minor disturbances may be overlooked during testing even with high-tech examination modalities. Yet, the ideal method of assessment is a case under dispute (6). Mechanoreceptive assessment techniques are static light touch detection, brush directional discrimination, two-point discrimination (sharp and blunt), and nociceptive ones are pin pressure nociception and thermal discrimination, both warm and cold (6). Two-point discrimination may differ in each patient, but the average value is considered around 5 mm. Yet, two-point discrimination is considered unreliable and is poorly reproductible owing to its very subjective nature (9). Several other authors rely on diagnostic nerve block as another method of subjective clinical sensorial assessment when the patient reports pain as a symptom. Consequently, if pain is relieved after the nerve block, a microneurosurgical intervention may offer a satisfactory prognosis for the patient (6).
Light touch sensation, pin-prick sensation and two-point discrimination are the preferred methods of functional assessment for LN injuries (10). Von Frey hair is usually used to evaluate the light touch sensation in a standardized fashion and the answers are scored on a four-point scale, 0 indicating no response and 3 indicating full response with no obvious difference from the contralateral side. Pain or sharpness is examined through the pin-prick test using a sharp probe with 15 g force. Two-point discrimination is performed with the patient’s eyes closed and tongue protruded. Both the affected and unaffected sites are tested on the tongue first to determine the minimum distance consistently reported as “two points”, which will set the threshold for that specific patient (10). On the other hand, according to authors’ clinical expertise, patients usually cannot discriminate the two points when the distance is under 12 mm on the tongue.
The Tinel’s sign may also serve as a useful tool during evaluation. It is defined as a tingling sensation or pain over the distribution of a nerve after applying pressure over the site of injury. Although it is originally developed for peripheral nerve testing in the limbs, it is used to evaluate injuries involving the LN, since it does not lie protected within a bony canal like the IAN (11). A positive sign is important because it may suggest a neuroma in-continuity and may aid in the decision for surgery (12).
There are also objective sensory tests which are the electrophysiological evaluation of the nerve with an encephalogram, monitorization of the orthodromic sensory nerve action through an electromyogram or the blink reflex, which is provoked by an electrical stimulation through electrodes (13-15).
The role of MRN
Despite exhibiting high positive and negative predictive values for LN injuries (93% and 100% respectively), NSTs show lower values for IAN injuries, with false-positive and false-negative rates of up to 23% and 40%, respectively. NST results are not reliable in the first month after the injury because of post-operative changes and the inability of patients and/or physicians to reproduce the sensory response. In addition, NST cannot determine the exact site of injury or delineate the anatomy for presurgical planning. MRN, an imaging dedicated to the peripheral nerves, provides a noninvasive map of neuromuscular anatomy and resolves the intraneural architecture in multiple orthogonal planes (16).
Currently, there are 2 different magnetic resonance imaging (MRI) methods available to study peripheral nerves: anatomic MRN and diffusion based functional MRN, particularly diffusion tensor imaging (DTI). MRN facilitates the detection of neuropathy by showing alterations of nerve caliber and abnormal intraneural T2 signal intensity ratio (T2SIR). DTI aids in the functional evaluation of the intraneural pathophysiology and altered diffusion characteristics correlate with axonal degeneration and demyelination (17).
In several studies, we have demonstrated that MRN have moderate to good correlations with the NST levels/Medical Research Council Scale (MRCS) grading scores and the surgical findings. The Sunderland Classification measured before surgery via NST matches with the surgical findings (n=26) in 58% of cases (15/26), overestimating the surgical findings in 8% of cases (2/26) and underestimating the surgical findings in 35% of cases (9/26). Overall, MRN provides valuable information about the status of the IAN and LN injuries and even post-repairs, which affect treatment decision and management (18).
The strength of MRN in patient management is the ability to provide non-invasive objective information of the IAN and LN that can distinguish normal from different degrees of neuropathy in the pre-injury, post-injury and post-repair phases. The correlation with surgical findings is moderate to good and if a randomized clinical trial can confirm this relationship with pre-surgical NST, then new strategies in patient management for IAN and LN should lead to quicker detection of non-recoverable injuries earlier than the current protocols and without dependence on patient’s response to clinical stimulus during NST testing, which should lead to improved outcomes of repair. Likewise, earlier detection of recoverable injuries should lead to risk reduction of unnecessary surgical interventions (18).
Factors influencing recovery
Final functional sensory recovery (FSR) is affected by age and pre-operative neurosensory function (8). Etiology, severity and mechanism of nerve injury, individual neuronal regeneration variability, the degree of neuronal inflammation/infection, cicatricial scar formation, experience of the surgeon, and the specific microneurosurgical techniques used in the repair also do affect the FSR outcomes.
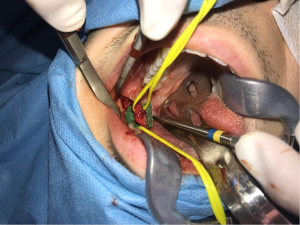
The authors emphasize three main principles of microneurosurgery for a successful repair including the access, preparation and microsurgery phases. The access phase allows the ability to complete the following phases, which overall significantly affect the outcome. During this first phase, exposure should always include normal nerve tissue on proximal and distal ends and a recommended 1 cm of exposure of nerve length is required to allow preparation and mobilization. An unobstructed view by adequate retraction and retention of adjacent tissues should be provided. Finally, the surgeon should ensure a surgical plane for the use of an operating microscope or loupes. The second phase is the preparation phase, aiming to identify normal nerve on the proximal and distal ends. This phase consists of the release of mesoneurium to explore the epineural tissues for pathologic tissues like a neuroma (Figure 1) or scar, the identification of normal fascicular pattern(s) from abnormal (e.g., intrafascicular scar), and if abnormal, the resection of nerve ends until normal nerve fascicles are identified through their white, plump and protruding look with interfascicular bleeding. Microsurgery is the third and final phase, aiming to approximate normal nerve tissues without any tension (19). Direct neurorrhaphy is indicated in case of a nerve gap of 5 mm or less whereas a gap larger than 5 mm ideally requires neurorrhaphy with a nerve graft (autograft, allograft or conduit).
Early vs. late repair
After Seddon’s publication regarding the factors influencing FSR, the timing from nerve injury to repair has gained significance and researchers began to investigate the effect of early intervention on the final outcomes.
For injuries to IAN or LN, 3 to 9 months are allowed for spontaneous nerve regeneration following the nerve injury, during which periodic monitoring of the patient is crucial. A surgical intervention is considered after this period, and if no FSR is observed (1).
Even the very early studies on nerve repair suggested that better outcomes are achieved within the first 6 months and the results of IAN and LN repair are less predictable after 6 months (20-22). More recent studies also suggest that to achieve a useful FSR after LN or IAN repair, surgical intervention is better performed within the 9 or 12 months for each nerve, respectively. It is also reported that with each month that pass, the odds of improvement decrease by 5.8% (23).
A recent meta-analysis reveals that an early repair has no significant effects on FSR in eight of the included studies, but four studies show a positive association between early repair and recovery. It is noteworthy to mention that the definitions of “early” and “late” differ between studies. Some consider the first 6 months as “early” whereas others consider three or even the first 2 months after injury as “early”. Overall, early repair seems to achieve better final FSR compared to delayed or late repair, although the specific time-period is ambiguous. Once the recovery proves to be below a level of FSR and the expectation is not towards spontaneous recovery, then timely surgical intervention may result in an improved outcome (1).
Nerve repair within the first 3 months is more likely to achieve FSR than a later intervention. Moreover, the 6-month time point is also significant to a lesser degree, compared to an even later repair (1).
Early repair, defined as repair completed within 90 days, results in earlier FSR than repairs done later (24). One systematic review on LN injuries supports the concept of early repair since it is associated with significantly increased probability of achieving FSR and is found to be the most significant prognostic factor in FSR outcomes. Although studies differ in their definitions for “early repair”, patients with LN injuries with indications for surgical intervention should receive treatment in an early interval. Borderline patients are harder to evaluate during the early phase for spontaneous recovery since this period is considered as three to 6 months, coinciding with the early surgical repair period (8).
Contradictory information hinders the proper decision making regarding the effect of time of repair on LN repair. Therefore, each surgeon should make a proper judgement during the decision-making process. Patients who seek treatment even after a prolonged observation period with no improvement may still benefit from repair, as long as the procedure is truly indicated (8). Authors believe that especially patients suffering from neuropathic pain usually clinically benefit even from delayed operations.
Certain algorithms for LN repair are proposed. A recent one by Atkins et al. focuses on LN injury following mandibular third molar removal and indicates surgery in cases of observed sectioning of LN intra-operatively, and dysesthesia or anesthesia post-operatively after 1 month if recovery is limited or absent (10).
Several other studies also report a trend towards better outcomes in the early repair although the results fail to reach statistical significance (24-26). One of these studies reveals that an early repair is 2.3 times more likely to result in FSR at 1 year but the outcome between early and late repair groups at final follow up show no difference (24). Yet there are several other studies failing to identify the time to repair as a significant factor influencing the neurosensory recovery (1,26-28).
Direct vs. indirect repair
Nerve grafts provide positive results in IAN and LN repairs (26,29). Some studies report better long-term results observed with nerve grafts, both objectively and subjectively, compared to direct nerve repair due to decreased tension at the repair site (27). Nerve regeneration can be negatively impacted by tension, which constricts the cross-sectional area of the fascicles of the nerve. This constriction increases the internal pressure thus compromising the intra fascicular nutritive blood flow (2,4). Preclinical studies have shown ischemia with as little as 5% elongation and impaired axonal regeneration with 7.4% nerve elongation. For the trigeminal nerve this is approximately 5 mm.
Excessive tension may be determined intra-operatively if both ends of the nerve fails to hold using a 9-0 nylon suture. Ideally, four to six sutures are placed depending on the nerve size. Placing an adequate number of sutures is advised since too many sutures may initiate more inflammatory reaction.
Autologous nerve grafts from sural nerve or the greater auricular nerve are commonly applied for IAN or LN repair, since they provide a good match for the number of fascicles (5). The supraclavicular, long thoracic, and cervical plexus nerves are also suggested (5,30-32). On the other hand, transverse cervical and lesser occipital nerves are considered less reliable than great auricular and supraclavicular nerves (5).
Autografts provide a suitable environment for nerve regeneration. Having no risk of an immunological reaction is the main advantage of an autograft, compared to an allograft which has an uncertain biocompatibility. Moreover, despite the need for a second surgical site, it is often simple, easy and safe to obtain an autograft (33). The main disadvantages of autografts are donor-site morbidity and limited availability (34). On the other hand, limited supply is not an issue with allografts which also bypass the need for additional surgical intervention for donor harvest. They therefore decrease both the operation time and potential donor site complications. However cost, potential immunoreaction and risk of disease transmission are major setbacks for allografts (5,35). Histologically, acellular grafts show similar axonal regeneration patterns as autografts (5).
There are also non-absorbable and absorbable non-biological conduits. Non-absorbable ones remain within the tissues and may initiate a foreign body reaction or cause irritation whereas the absorbable ones function as temporary scaffolds (5). Conduits from autogenous vein grafts show successful results for gaps of less than 5mm in LN reconstruction and for gaps ranging between 5–14 mm in IAN repair (36).
The current knowledge fails to show any difference between IAN or LN for primary reconstruction (7). There is a risk of graft collapse with the movement of the tongue in LN, which is not a concern for IAN since it is located within a bony canal (36). On the other hand, since the LN is not constricted within a bony canal, it can be further mobilized by dissection. Within studies where any difference in FSR is observed between IAN and LN repair, this may be credited to the inability to adequately mobilize the IAN and ensure a tension free repair, and not because there is an inherent difference in nerve recovery (7).
In a systematic review to assess the outcomes of direct LN repair, six studies were fit to be analyzed. British Medical Research Council (BMRC) criteria was accepted for all six studies, which helped to standardize the level of recovery after the surgical intervention. Accordingly, FSR was determined as S3 or higher on BMRC scale. Consequently, the review failed to reveal a significant relationship between FSR and conduit use (8). Authors believe a greater number of randomized clinical trials and prospective studies are necessary to draw definitive lines regarding the positive effects of conduit use on FSR of IAN and LN repairs.
Management
It is important to understand the overwhelming aspect of a nerve injury on the patient’s quality of life and accordingly provide a psychological treatment as well, through immediate information, explanation, support and reassurance of realistic expectations from the treatment (6,37).
There is still need for a clear consensus on how IAN or LN should be evaluated post-operatively (9). Neurosensorial assessments are performed through fine touch, pin-prick pain, two-point discrimination, thermal testing, taste testing and fungiform papillae examination in LN injury cases (1).
Initially, acute nerve injury treatment starts with medical therapy. Pharmacologically, an acute nerve injury may benefit from corticosteroids or non-steroid anti-inflammatory drugs (NSAIDs). High doses of adrenocorticosteroids within the first week of injury is considered useful in minimizing neuropathy as well as inhibiting neuroma formation (6,38). Topical application of dexamethasone (in intravenous form) helps reduce neural inflammation and enhance recovery, when a known or observed trauma is witnessed intra-operatively (39). However, it is crucial to either indicate a microneurosurgical intervention or to refer patients to a microneurosurgeon when IAN transection is observed intra-operatively (6). Antidepressants, anticonvulsants, antisympathetic drugs or physiological therapies such as transcutaneous electric nerve stimulation, acupuncture, cryotherapy and low-level laser therapy may also be considered (6).
If a NSD exists post-operatively after a week, examinations should be continued weekly for 3 weeks followed by every 2 to 3 weeks for 12 weeks (6).
One theory is that successful surgical intervention is most predictable when performed before total Wallerian degeneration of the distal portion of the nerve has occurred, which is approximately 3 months or, for several other authors, may take up to 4 to 6 months, due to the slow degeneration process of the nerve (6,40).
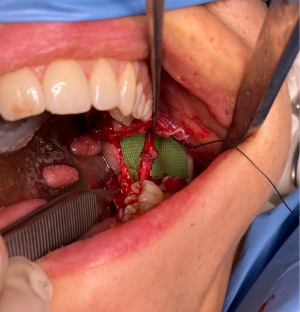
The surgical management of peripheral nerve injuries range from external neurolysis to direct microsuturing, gluing, grafting, tubulization, and laser welding (41). If tension-free repair is achievable, direct neurorrhaphy (Figure 2) should be indicated. Even though its limited applicability, additional dissection and mobilization of a nerve may be beneficial to minimize or eliminate a nerve gap (7). Juodzbalys et al. suggest that the best results are obtained with a direct anastomosis of the two ends of the nerve to be repaired (6). However, this may be impractical especially in mandibular resection operations which often result in IAN discontinuity. In such cases, nerve-sparing techniques as well as immediate reconstruction using autogenous nerve grafts or nerve allografts have been reported (42,43).
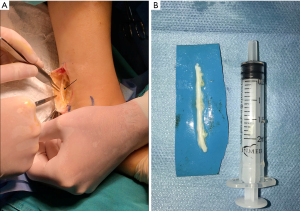
Besides an end-to-end repair, other techniques including decompression through external and internal neurolysis, excision and direct or indirect anastomosis using an autogenous sural, greater auricular or medial antebrachial nerve graft, saphenous vein graft, or alloplastic, collagen or polyglycolic acid tubes show variable results (6). When direct neurorrhaphy is impracticable, grafts or conduits are used to bridge the nerve gap (7). Autologous nerve graft (Figure 3) is considered the gold standard for the repair of a long nerve gap, even though it constitutes disadvantages such as the need for a second surgical site, scarring, donor site morbidity, neuroma formation, loss of sensitivity at the donor site and limited availability (7,41).
Alternatively, tubulization (Figure 4) provides a favorable microenvironment through a conduit inside of which cellular components and growth factors can be added to enhance nerve regeneration (41). These conduits may either be synthetic (non-resorbable, silicone) or resorbable (type I collagen, polyglycolic acid, or porcine intestinal submucosa) (7). Their use for nerve reconstruction of gaps larger than 6 mm is not advisable. Alternatively, conduits may aid in promoting nerve regeneration after direct neurorrhaphy of nerve gaps less than 6 mm (7,44). Despite their limited efficacy in nerve gaps, they are advocated as adjuncts, to either primary repairs or allograft reconstructions, as nerve wraps or connector-assisted repairs around the coaptation site (7).
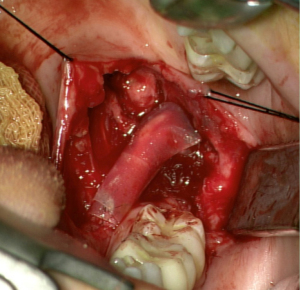
Processed nerve allografts (PNAs) help avoid donor-site complications due to autograft harvesting. Human PNAs (Figure 4) are extracellular matrix scaffolds created from donated human peripheral nerve tissues that has been pre-degenerated, decellularized, and sterilized (7).
Some studies report that a tension-free coaptation with a conduit or connector is associated with less sensory disturbance than direct neurorrhaphy for both IAN and LN reconstructions, providing 95% and 89% FSR rates, respectively (7,45).
If IAN injury is encountered after implant surgery, removal of the implant may be considered within the 36 hours, post-operatively, when a contact with or pressure is suspected onto the mandibular canal (46). Nonetheless, removal of the implant may not always resolve any injuries especially when the injury may be related to other sources than impingement such as inadvertent drilling through the IAN. The surgeon should carefully monitor the patient, evaluate through serial NSTs or consider getting an MRN.
Although the literature suggests several ways to manage an already osseointegrated implant either through its removal or by apicoectomy of the implant, these are not very practical methods to use (6). The authors suggest to leave the implant and instead repair the nerve apical to the implant using standard techniques.
Similarly, processed and acellular nerve allografts have promising but variable results. We have previously reported that FSR is achieved in 90% of patients after a mandibular resection surgery, involving the reconstruction of IAN with a PNA (42). On the other hand, the main setback for an acellular nerve graft (ANG) so far has been its inability to effectively reconstruct nerve gaps larger than 20 mm (29,42). Yet, PNAs are not recommended for nerve gaps longer than 3 cm in non-trigeminal sites and the immediate reconstruction of IAN with PNA in long gaps (45–70 mm) have resulted in functional sensory impairment in small-diameter (2–3 mm) nerves (42).
Either neuroma-in-continuity or proximal terminal neuroma may be encountered as a serious complication following LN injury which may be managed with a variety of different techniques including end-to-end anastomosis, or indirect repair using autograft or allograft (10). Several studies favor end-to-end anastomosis since they result in the best outcome, advocating that intraneural scar formation is a major setback against neural recovery and a single repair site may result in better outcomes (10,47). On the other hand, grafting may be a better choice when the nerve gap is large and possibly increase the tension on the repair (10,27).
Special considerations on the IAN
IAN injury, similar to LN injury, may commonly arise due to iatrogenic causes such as third molar extractions, dental implant placement, endodontic treatments, orthognathic surgery, dentoalveolar pathology, and even because of anesthetic needles or the toxic effects of the anesthetic agents themselves. IAN injury is one of the serious complications of implant surgery with an incidence of up to 40% (6).
Clinically, IAN injury may result in an altered sensation, such as anesthesia, paresthesia or dysesthesia, with or without pain in the ipsilateral lower lip, mucous membrane and gingiva until the second premolar as well as the skin of the mental area. Daily tasks including speaking, eating, drinking, kissing, applying make-up or shaving may be affected (6).
Complete or partial transection, extension, compression, crushing or ischemia of the IAN may have a clinical presentation of nerve damage (9). Common risk factors for IAN injury are older age and female gender and although injury due to local anesthesia is very rare, it is also a major concern for IAN injury. Almost up to 9% of patients experience an “electric shock” during an IAN block application of which 57% suffer further from prolonged neuropathy (6). Luckily, these commonly resolve on their own within 8 weeks without a need for a surgical intervention, but may benefit from medical treatments. As far as implant surgery is concerned, several authors advocate the use of local infiltration instead of an IAN block, even though it is not generally used due to the nerve endings within the bone which may cause discomfort during surgery (6). The authors strongly advise not to rely on local infiltrative anesthesia in the prevention of IAN injury as they have experienced numerous cases being referred with IAN injuries during dental implant operations performed using only infiltrative local anesthesia instead of regional mandibular blocks. Chemical properties of a local anesthetic agent may also be considered as a possible etiological factor for IAN injury. Several studies advocate that prilocaine (4%) and articaine (4%) are more prone to such injury, per use, than lidocaine (6,48). This is attributed to the degree of inflammatory reaction to the local anesthetic since lidocaine is reportedly less irritant than articaine (49). Overall, demyelination, axonal degeneration and inflammation of the surrounding nerve fibers within the fascicles are listed as the main factors resulting in a chemical injury (50). Ideally, it is recommended to contact each patient after the proposed effect of local anesthesia has worn of, approximately 6 hours after the surgery to make sure that the patient does not have an ongoing anesthesia or a neuropathic condition (46).
Specific to implant surgery, injuries due to implant drill or the dental implant, and an incorrect surgical technique are listed as the main etiological factors. Additionally, an IAN injury may occur either intra-operatively (mechanical, thermal or chemical injuries), or post-operatively (indirect injury due to thermal stimuli, infection or hematoma resulting in scarring and ischemia). Mechanical etiological factors, the implant drill or the implant itself, may either have a direct or indirect effect on IAN, consequently causing ischemia or nerve degeneration. The implant or the drill or may partially/fully intrude the mandibular canal, or cause a thermal stimulus evoking bony necrosis (6).
Some authors propose that even when the roof the mandibular canal is cracked, hemorrhage and debris deposits may compress and result in an ischemia of the IAN and the injury may persist even when the implant is backed-up or replaced with a shorter one (46). Post-operative infection or peri-implant lesions may also result in an IAN injury, as well as iatrogenic injuries due to incorrect surgical technique (6).
IAN damage may also occur due to SSRO (9). Nerve damage may result from compression or movement by bone fragments, direct mechanical stimulation of the nerve or an indirect damage (51). The incidence of IAN damage after SSRO ranges between 0–85% in the literature (9). Persistent IAN hypoesthesia is the most commonly reported complication after SSRO (52).
Nerve damage may occur at different stages during SSRO, such as during the dissection of soft tissues medial to the mandibular ramus, mandibular sawing, splitting, advancement or fragment fixation. Interestingly, split type is not found to affect the rate of post-operative neurosensory disturbance (9). Age, type of fixation, surgical technique and experience, magnitude of mandibular movement and the position of the IAN are the main risk factors of neurosensory disturbance after SSRO (52).
IAN lateralization and repositioning may also result in neurosensory complications in 95.5% and 58.9% of patients, 3.4% and 22.1% of which experience a permanent injury, respectively (53).
An incorrect surgical technique may result in an IAN injury due to the wrong use of scalpel or sutures, and improper use of reflectors or false soft tissue reflection. Although these factors usually result in direct nerve injury, IAN injury may also be encountered because of soft tissue edema post-operatively after an improper operation technique (6).
Special considerations on the LN
Running subperiosteally at the lingual aspect of the posterior mandible, the LN anatomically has a close relation with mandibular third molars, extractions of which are one of the most common surgical procedures (54,55). Iatrogenic LN injuries occur most frequently due to mandibular third molar extractions. Patients experience temporary or permanent paresthesia in 4.4% and 1% of cases, which annually translate into 154,000 and 35,000 patients, respectively (7). The risk of LN injury is greater during mandibular third molar extractions where distobuccal bone removal is performed (55). Improper instrument placement during lingual retraction and excessive retraction also increase the incidence of LN injury (56).
Clinically, LN injury may cause loss of sensation to the ipsilateral anterior tongue and lingual mucoperiosteum (55). Other symptoms may include neuropathic pain (most likely due to neuroma formation), reduced or altered taste, impaired speech or unintentional tongue biting (10). Patients experiencing such symptoms should be informed that without an intervention, those with no signs of significant resolution by 3 months are unlikely to recover spontaneously (10).
Surgical intervention for LN repair 6 months after the injury may have a higher risk of neuropathic pain or may offer no difference (57). Yet, the majority of patients with initial symptoms of neuropathic pain are relieved from this pain after surgical intervention. Even though significant improvements are observed in terms of recovery, Atkins et al. suggest that no patient experiences a complete recovery. Still, surgery seems to be the most worthwhile option for patients with pain (10).
A surgical intervention for LN injury is considered when there is significant anesthesia or neuropathic pain in the form of dysesthesia or hyperalgesia. A significantly less incidence of tongue-biting is observed in patients after surgical intervention which may be attributed to an improved pin-prick detection (10). Other surgical outcomes may be listed as significantly reduced threshold during two-point discrimination and an improved ability to detect light touch (10).
There are contradictory reports regarding how taste is affected after the surgical repair of the LN. Several authors report a lack of improvement in taste whereas others show variable degrees of improvement in gustatory function (10). Nakanishi et al. suggests that poorer outcomes in taste may be associated with delayed surgery (28).
Although there are special instruments designed specifically for lingual flap retraction, such as the Hovell, Walter, and Rowe elevators, many surgical instruments are used beyond their initial purpose (58). The Howarth periosteal elevator, which is originally designed as a nasal mucoperiosteal elevator, is in fact a good example for such repurposed instruments, since it is most commonly used for lingual retraction. Similarly, the Freer, Molt, and Obwegeser narrow periosteal elevators are repurposed as lingual retractors (55). One study comparing the use of no lingual retractors, repurposed and purpose-built lingual retractors reveal that the risk of LN injury is increased when repurposed retractors are used than with no lingual retractor. Using a purpose-built instrument provides the lowest risk of injury (55).
When the extractions are done by fracturing the lingual plate of the alveolar socket, also known as the lingual split, there is a higher incidence of temporary injury but a lower incidence of permanent damage than lingual retraction alone (56). Therefore, Rapaport et at. suggests that although permanent nerve injuries are uncommon, using purpose-built lingual flap retractors may help avoid the overwhelming consequences for the patients (55).
Conclusions
Damage to the inferior alveolar or LNs may have devastating consequences both for the patients and the physicians. It is important to consider the etiological factors and make an appropriate pre-operative assessment to avoid nerve injury. Even if it happens, a timely diagnosis and a proper management are key to avoid further or permanent damage.
Although the literature supports the tendency towards the concept of management as “earlier is better”, delayed repairs also show acceptable neurosensory recovery outcomes (1). Within the first 3 months after injury, nerve repair for IAN and LN has a better chance of achieving FSR than a later intervention. Although it is significant to a lesser degree, the 6-month time point also differs than an even later repair (1).
For LN repairs, conduits seem to have a positive effect on neurosensory recovery and early repair is highly correlated with increased FSR compared to late repairs (8). Currently, autografts and allografts, as well as primary tension-free repairs are reportedly found superior to conduits in achieving FSR. Conduits may prove to be beneficial in nerve gaps of 6 mm or less, however this information is yet to be further evaluated through newer studies with larger sample sizes. Within autografts, allografts and primary repairs, no statistical differences are observed since they have all achieved FSR at comparable rates (7).
Patients should be advised to avoid self-inflicted trauma in the immediate post-operative period when hypoesthesia may initially be increased. Jaw opening physiotherapy is advised after LN repair due to stripping of the medial pterygoid during surgical exposure. Patients can begin sensory re-training immediately post-operatively using a toothbrush to stimulate sensory nerves on the tongue or a cotton tip on the lip and chin in front of a mirror (59).
Serial formal sensory exams should begin at the earliest post-operative 1 month (preferably on the third month) and continue monthly assessing for improvement up to a year. If patients fail to improve at 3 to 6 months post-repair, a repeat MRN can be completed to assess formation of secondary neuromas which may require a second surgery.
Innovative research on nerve repair focuses on further optimizing clinical outcomes. One of the future trends for nerve repair will be the third-generation conduits, which are currently under development. These will incorporate stem cells, Schwann cells or extracellular matrix proteins, and allow controlled delivery of neurotrophic factors for guided regrowth (7,60). Similarly, allograft modifications including nerve growth promoting factors or the application of electric or magnetic stimulation are among future considerations (7).
Finally, even though there are promising clinical studies on the use of preparation rich in growth factor (PRGF), platelet-rich fibrin (PRF) or platelet-rich plasma (PRP) as adjunctive methods for nerve repair, the clinical data on their use is limited and many reports are based on animal studies (41,52,53,61). New prospective studies are necessary to evaluate the possibly positive effects of these autologous blood concentrates as well as hyperbaric oxygen therapy as adjunctive methods for nerve repair.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sung-Kiang Chuang) for the series “Clinical Outcomes and Innovations in Oral and Maxillofacial Surgery” published in Frontiers of Oral and Maxillofacial Medicine. The article has undergone external peer review.
Peer Review File: Available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm-21-8/coif). The series “Clinical Outcomes and Innovations in Oral and Maxillofacial Surgery” was commissioned by the editorial office without any funding or sponsorship. JRZ is a paid consultant for AxoGen, Inc. and his contributions to this publication were not supported by or reviewed by AxoGen, Inc. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Suhaym O, Miloro M. Does early repair of trigeminal nerve injuries influence neurosensory recovery? A systematic review and meta-analysis. Int J Oral Maxillofac Surg 2021;50:820-9. [Crossref] [PubMed]
- SUNDERLAND S.. A classification of peripheral nerve injuries producing loss of function. Brain 1951;74:491-516. [Crossref] [PubMed]
- Althagafi A, Nadi M. Acute Nerve Injury. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
- Miloro M. Microneurosurgery. In: Miloro M, Ghali GE, Larsen P, et al. Peterson's Principles Of Oral & Maxillofacial Surgery. 3rd edition. Connecticut, USA: PMPH-USA, 2011:919-41.
- Liu X, Daugherty R, Konofaos P. Sensory Restoration of the Facial Region. Ann Plast Surg 2019;82:700-7. [Crossref] [PubMed]
- Juodzbalys G, Wang HL, Sabalys G. Injury of the Inferior Alveolar Nerve during Implant Placement: a Literature Review. J Oral Maxillofac Res 2011;2:e1. [Crossref] [PubMed]
- Ducic I, Yoon J. Reconstructive Options for Inferior Alveolar and Lingual Nerve Injuries After Dental and Oral Surgery: An Evidence-Based Review. Ann Plast Surg 2019;82:653-60. [Crossref] [PubMed]
- Kogan M, Lee KC, Chuang SK, et al. Outcomes of Direct Lingual Nerve Repair After an Injury: A Systematic Review. J Oral Maxillofac Surg 2021;79:697-703. [Crossref] [PubMed]
- Martinez-de la Cruz G, Yamauchi K, Saito S, et al. The relationship between neurosensory disturbance of the inferior alveolar nerve and the lingual split pattern after sagittal split osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol 2020;130:373-8. [Crossref] [PubMed]
- Atkins S, Kyriakidou E. Clinical outcomes of lingual nerve repair. Br J Oral Maxillofac Surg 2021;59:39-45. [Crossref] [PubMed]
- Nerve Evaluation Protocol 2014. California Association of Oral and Maxillofacial Surgeons, 2014.
- Donoff RB, Fagin AP. Lingual and inferior alveolar nerve injuries after third molar removal. Alpha Omegan 2013;106:91-5. [PubMed]
- Nakagawa K, Ueki K, Takatsuka S, et al. Somatosensory-evoked potential to evaluate the trigeminal nerve after sagittal split osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:146-52. [Crossref] [PubMed]
- Jääskeläinen SK, Teerijoki-Oksa T, Forssell K, et al. Intraoperative monitoring of the inferior alveolar nerve during mandibular sagittal-split osteotomy. Muscle Nerve 2000;23:368-75. [Crossref] [PubMed]
- Jääskeläinen SK. Blink reflex with stimulation of the mental nerve. Methodology, reference values, and some clinical vignettes. Acta Neurol Scand 1995;91:477-82. [Crossref] [PubMed]
- Dessouky R, Xi Y, Zuniga J, et al. Role of MR Neurography for the Diagnosis of Peripheral Trigeminal Nerve Injuries in Patients with Prior Molar Tooth Extraction. AJNR Am J Neuroradiol 2018;39:162-9. [Crossref] [PubMed]
- Thawait SK, Chaudhry V, Thawait GK, et al. High-resolution MR neurography of diffuse peripheral nerve lesions. AJNR Am J Neuroradiol 2011;32:1365-72. [Crossref] [PubMed]
- Zuniga JR, Mistry C, Tikhonov I, et al. Magnetic Resonance Neurography of Traumatic and Nontraumatic Peripheral Trigeminal Neuropathies. J Oral Maxillofac Surg 2018;76:725-36. [Crossref] [PubMed]
- Tay AB, Zuniga J. Nerve Repair. In: Haggerty CJ, Laughlin RM, editors. Atlas of operative oral and maxillofacial surgery. John Wiley & Sons, 2015:522-34.
- Mozsary PG, Middleton RA. Microsurgical reconstruction of the lingual nerve. J Oral Maxillofac Surg 1984;42:415-20. [Crossref] [PubMed]
- Mozsary PG, Syers CS. Microsurgical correction of the injured inferior alveolar nerve. J Oral Maxillofac Surg 1985;43:353-8. [Crossref] [PubMed]
- Donoff RB. Surgical management of inferior alveolar nerve injuries (Part I): The case for early repair. J Oral Maxillofac Surg 1995;53:1327-9. [Crossref] [PubMed]
- Bagheri SC, Meyer RA, Khan HA, et al. Retrospective review of microsurgical repair of 222 lingual nerve injuries. J Oral Maxillofac Surg 2010;68:715-23. [Crossref] [PubMed]
- Susarla SM, Kaban LB, Donoff RB, et al. Does early repair of lingual nerve injuries improve functional sensory recovery? J Oral Maxillofac Surg 2007;65:1070-6. [Crossref] [PubMed]
- Strauss ER, Ziccardi VB, Janal MN. Outcome assessment of inferior alveolar nerve microsurgery: a retrospective review. J Oral Maxillofac Surg 2006;64:1767-70. [Crossref] [PubMed]
- Zuniga JR. Sensory outcomes after reconstruction of lingual and inferior alveolar nerve discontinuities using processed nerve allograft--a case series. J Oral Maxillofac Surg 2015;73:734-44. [Crossref] [PubMed]
- Miloro M, Ruckman P 3rd, Kolokythas A. Lingual Nerve Repair: To Graft or Not to Graft? J Oral Maxillofac Surg 2015;73:1844-50. [Crossref] [PubMed]
- Nakanishi T, Yamamoto Y, Tanioka K, et al. Effect of duration from lingual nerve injury to undergoing microneurosurgery on improving sensory and taste functions: retrospective study. Maxillofac Plast Reconstr Surg 2019;41:61. [Crossref] [PubMed]
- Yampolsky A, Ziccardi V, Chuang SK. Efficacy of Acellular Nerve Allografts in Trigeminal Nerve Reconstruction. J Oral Maxillofac Surg 2017;75:2230-4. [Crossref] [PubMed]
- Mucci SJ, Dellon AL. Restoration of lower-lip sensation: neurotization of the mental nerve with the supraclavicular nerve. J Reconstr Microsurg 1997;13:151-5. [Crossref] [PubMed]
- Audolfsson T, Rodríguez-Lorenzo A, Wong C, et al. Nerve transfers for facial transplantation: a cadaveric study for motor and sensory restoration. Plast Reconstr Surg 2013;131:1231-40. [Crossref] [PubMed]
- Schultes G, Gaggl A, Kärcher H. Transplantation of the vascular pedicled thoracicus longus nerve for recovering sensory capacities in the mentalis nerve supply region. Mund Kiefer Gesichtschir 1999;3:1-5. [Crossref] [PubMed]
- Gao Y, Wang YL, Kong D, et al. Nerve autografts and tissue-engineered materials for the repair of peripheral nerve injuries: a 5-year bibliometric analysis. Neural Regen Res 2015;10:1003-8. [Crossref] [PubMed]
- Trehan SK, Model Z, Lee SK. Nerve Repair and Nerve Grafting. Hand Clin 2016;32:119-25. [Crossref] [PubMed]
- Siemionow M, Sonmez E. Nerve allograft transplantation: a review. J Reconstr Microsurg 2007;23:511-20. [Crossref] [PubMed]
- Pogrel MA, Maghen A. The use of autogenous vein grafts for inferior alveolar and lingual nerve reconstruction. J Oral Maxillofac Surg 2001;59:985-8; discussion 988-93. [Crossref] [PubMed]
- Abarca M, van Steenberghe D, Malevez C, et al. Neurosensory disturbances after immediate loading of implants in the anterior mandible: an initial questionnaire approach followed by a psychophysical assessment. Clin Oral Investig 2006;10:269-77. [Crossref] [PubMed]
- Han SR, Yeo SP, Lee MK, et al. Early dexamethasone relieves trigeminal neuropathic pain. J Dent Res 2010;89:915-20. [Crossref] [PubMed]
- Misch C. Root Form Surgery in the Edentulous Anterior and Posterior Mandible: Implant Insertion. In: Misch C. Contemporary Implant Dentistry. 3rd edition. St. Louis, MO: Elsevier Health Sciences, 2008:221-6.
- Kraut RA, Chahal O. Management of patients with trigeminal nerve injuries after mandibular implant placement. J Am Dent Assoc 2002;133:1351-4. [Crossref] [PubMed]
- Bastami F, Vares P, Khojasteh A. Healing Effects of Platelet-Rich Plasma on Peripheral Nerve Injuries. J Craniofac Surg 2017;28:e49-57. [Crossref] [PubMed]
- Zuniga JR, Williams F, Petrisor D A. Case-and-Control, Multisite, Positive Controlled, Prospective Study of the Safety and Effectiveness of Immediate Inferior Alveolar Nerve Processed Nerve Allograft Reconstruction With Ablation of the Mandible for Benign Pathology. J Oral Maxillofac Surg 2017;75:2669-81. [Crossref] [PubMed]
- Salomon D, Miloro M, Kolokythas A. Outcomes of Immediate Allograft Reconstruction of Long-Span Defects of the Inferior Alveolar Nerve. J Oral Maxillofac Surg 2016;74:2507-14. [Crossref] [PubMed]
- Lohmeyer JA, Kern Y, Schmauss D, et al. Prospective clinical study on digital nerve repair with collagen nerve conduits and review of literature. J Reconstr Microsurg 2014;30:227-34. [PubMed]
- Ducic I, Safa B, DeVinney E. Refinements of nerve repair with connector-assisted coaptation. Microsurgery 2017;37:256-63. [Crossref] [PubMed]
- Khawaja N, Renton T. Case studies on implant removal influencing the resolution of inferior alveolar nerve injury. Br Dent J 2009;206:365-70. [Crossref] [PubMed]
- Zuniga JR, Yates DM. Factors Determining Outcome After Trigeminal Nerve Surgery for Neuropathic Pain. J Oral Maxillofac Surg 2016;74:1323-9. [Crossref] [PubMed]
- Hillerup S, Jensen R. Nerve injury caused by mandibular block analgesia. Int J Oral Maxillofac Surg 2006;35:437-43. [Crossref] [PubMed]
- Ribeiro PD Jr, Sanches MG, Okamoto T. Comparative analysis of tissue reactions to anesthetic solutions: histological analysis in subcutaneous tissue of rats. Anesth Prog 2003;50:169-80. [PubMed]
- Malamed S. What's new in local anaesthesia? SAAD Dig 2009;25:4-14. [PubMed]
- Yamauchi K, Takahashi T, Kaneuji T, et al. Risk factors for neurosensory disturbance after bilateral sagittal split osteotomy based on position of mandibular canal and morphology of mandibular angle. J Oral Maxillofac Surg 2012;70:401-6. [Crossref] [PubMed]
- Tabrizi R, Pourdanesh F, Jafari S, et al. Can platelet-rich fibrin accelerate neurosensory recovery following sagittal split osteotomy? A double-blind, split-mouth, randomized clinical trial. Int J Oral Maxillofac Surg 2018;47:1011-4. [Crossref] [PubMed]
- Anitua E, Alkhraisat MH. The adjuvant use of plasma rich in growth factors in the inferior alveolar nerve repositioning technique. Heliyon 2019;5:e02965. [Crossref] [PubMed]
- Baqain ZH, Abukaraky A, Hassoneh Y, et al. Lingual nerve morbidity and mandibular third molar surgery: a prospective study. Med Princ Pract 2010;19:28-32. [Crossref] [PubMed]
- Rapaport BHJ, Brown JS. Systematic review of lingual nerve retraction during surgical mandibular third molar extractions. Br J Oral Maxillofac Surg 2020;58:748-52. [Crossref] [PubMed]
- Pichler JW, Beirne OR. Lingual flap retraction and prevention of lingual nerve damage associated with third molar surgery: a systematic review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:395-401. [Crossref] [PubMed]
- Renton T, Yilmaz Z, Gaballah K. Evaluation of trigeminal nerve injuries in relation to third molar surgery in a prospective patient cohort. Recommendations for prevention. Int J Oral Maxillofac Surg 2012;41:1509-18. [Crossref] [PubMed]
- Pogrel MA, Goldman KE. Lingual flap retraction for third molar removal. J Oral Maxillofac Surg 2004;62:1125-30. [Crossref] [PubMed]
- Phillips C, Blakey G 3rd, Essick GK. Sensory retraining: a cognitive behavioral therapy for altered sensation. Atlas Oral Maxillofac Surg Clin North Am 2011;19:109-18. [Crossref] [PubMed]
- Gaudin R, Knipfer C, Henningsen A, et al. Approaches to Peripheral Nerve Repair: Generations of Biomaterial Conduits Yielding to Replacing Autologous Nerve Grafts in Craniomaxillofacial Surgery. Biomed Res Int 2016;2016:3856262. [Crossref] [PubMed]
- Sánchez M, Garate A, Delgado D, et al. Platelet-rich plasma, an adjuvant biological therapy to assist peripheral nerve repair. Neural Regen Res 2017;12:47-52. [Crossref] [PubMed]
Cite this article as: Selvi F, Yildirimyan N, Zuniga JR. Inferior alveolar and lingual nerve injuries: an overview of diagnosis and management. Front Oral Maxillofac Med 2022;4:27.

