
Risk factors for distant metastasis in oral cancer and a strategy preoperative detection
Introduction
Distant metastasis (DM) from oral carcinoma is usually defined as dissemination of the disease to organs or tissues below the level of the clavicles (1). DM plays a critical role in the management and prognosis of oral squamous cell carcinoma (OSCC). Specifically, overall survival dramatically decreases in cases where distant dissemination is evidenced at presentation or during follow-up (2). With regard to DM from oral cancer, the base of the tongue is the most commonly affected primary site followed by anterior tongue, floor of the mouth, buccal mucosa, and maxilla. The lung is the organ most frequently involved, followed by bone, skin, liver and brain. According to several reports, the incidence of DM at presentation ranges between approximately 1–3% (3,4). However, this value could increase up to 15% during follow-up. Hence, the creation of an accurate protocol based on the assessment of risk factors could be extremely useful for identifying high risk patients. Several clinical and histopathological features have been linked to the development of distant metastases, from oral cavity squamous cell carcinoma (SCC) such as T-stage, N-stage, extracapsular invasion, and tumor thickness (TT) (5). The main aim of the present report is to identify the factors most strongly related to the development of DM in patients affected by OSCC, and to define a protocol for the early detection of DM.
Methods
Between 2009 and 2017, 297 previously untreated patients with OSCC were diagnosed and treated with surgery ± adjuvant treatment at the Hospital General Universitario of Albacete (Spain). Patients with oropharyngeal SCC were not included in this study. All patients presented a positive biopsy for OSCC, and a computed tomography (CT) scan of the cervicofacial area was conducted in all patients to allow for the accurate clinical staging of the disease before performing ablative surgery. Postoperative histopathological examination confirmed the diagnosis of OSCC in all cases. The clinical and pathological stage of the primary tumor was initially determined by using the recommendations of the fifth edition of the Union for International Cancer Control (UICC) TNM classification of malignant tumors because it was the classification commonly used by the pathology department of our institution at when the patients analyzed in this study underwent surgery (6). However, in this retrospective study, the clinical and pathological stage of each patient was reconsidered according to the eighth edition of the UICC TNM classification of malignant tumors (7). Several pathological features, such as T-stage, N-stage, TT, perineural invasion, vascular invasion, extracapsular spread (ECS), recurrence, involvement of contralateral neck, pathological stage, age and sex were also considered. Three categories of TT were established: <0.5, 0.5–1 and >1 cm. Age was also divided into three categories: <40, 40–60 and >60 years.
Ethical approval is not required by our institution for retrospective study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Statistical analysis
Statistical analysis was conducted using SPSS 23v. A correlation test was conducted to analyze the relationships between variables. Frequencies and percentages were used to evaluate the distribution of variables such as sex and age, along with the value and distribution of DM. A Chi-square test was conducted to compare the differences between patients with DM and those without DM, and specific contingency tables allowed for calculating the impact of each variable on the risk of developing DM. A Cox regression analysis was also conducted to verify the relationship and the hazard ratio (HR) of each variable with DM. The P value was set at 0.05. Finally, a Kaplan-Meier test was applied to obtain an overall 5-year survival analysis.
Inclusion criteria
Patient with a positive preoperative biopsy for OSCC underwent ablative surgery in our institution, patients with OSCC that developed DM during follow-up, patients with a complete radiological preoperative study, including magnetic resonance imaging (MRI) or CT scan of the cervicofacial area, before surgery.
Exclusion criteria
Patients with SCC of the oropharynx, patients with evidence of DM before surgery.
Variables
T-stage, N-stage, TT, perineural invasion, vascular invasion, ECS, recurrence, involvement of contralateral neck, pathological stage, age and survival represent the variables analyzed in this study.
Results
Two hundred and ninety-seven patients underwent surgical ablation of OSCC ± adjuvant treatment in the preestablished period. The sample consisted of 206 males (69.4%) and 91 females (30.6%) with a M/F ratio of 2.09. The age of the participants ranged between 29 to 92 years (mean: 63.97±11.80 years). The mean follow-up time was 23.83±15.09 months (range, 3–71 months).
The incidence of DM was 13.5% (n=40). The most common site of metastasis was the lung (n=25; 62.5%), followed by bone (n=3; 7.5%), liver (n=2; 5.0%), and adrenal glands (n=2; 5.0%). In addition, seven patients showed multiple metastases (17.5%) and one patient suffered carcinomatous lymphangitis (n=1; 2.5%). DM was evident during the first year of follow-up in 19 patients (47.5%) and during the second year in seven patients (17.5%). However, 35.0% of patients (n=14) were diagnosed during the first 3 months following surgery. In these patients, DM was evidenced with chest CT or positron emission tomography (PET) scan performed before beginning or just after finishing adjuvant treatment. Therefore, 4.7% of our sample (14/297) showed an early presentation of DM and we are thus unable to rule out the possibility that these patients had already presented distant dissemination of the disease before performing the ablative surgery, and that DM had been undetected by our staging protocol (clinical exploration + CT scan of the cervicofacial area).
Regarding the risk factors associated with DM, T3 and T4 stage (P<0.001), N2 and N3 stage (P<0.001), TT >1 cm (P<0.001), perineural invasion (P<0.001), ECS (P<0.001), cervical and locoregional recurrence (P<0.001), clinical or pathological stage IV (P<0.001) and involvement of contralateral neck (P<0.005) were strongly associated with the development of DM. Interestingly, vascular invasion, age and sex were not associated with the risk of developing distant metastases (P>0.005). Further details regarding the specific value of each subgroup and its relationship with the risk of developing DM are displayed in Table 1.
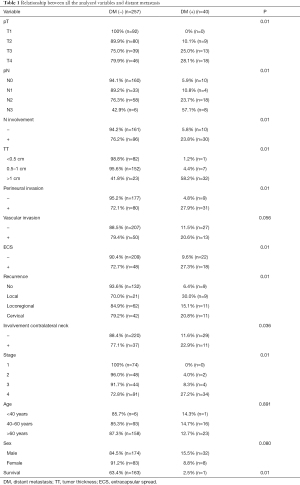
Full table
According to the results of the Cox regression analysis, the risk of developing DM is 2.227 times higher in patients with pT3 or pT4 tumors, 3.123 times higher in patients with N2 or N3, 1.9 times higher in patients with TT >1 cm, 3.082 times higher in patients with cervical or locoregional recurrence, and 2.651 times higher in patients with clinical or pathological stage IV. Further details about the specific HR value are described in Table 2. In addition, the possibility of developing distant metastases is more than 4 times greater when two or more of these risk factors are present.
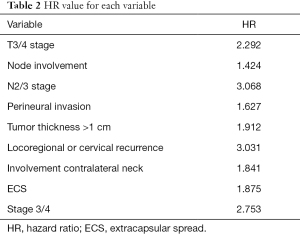
Full table
Importantly, overall survival was 63.4% in patients without evidence of DM at follow-up, and only 2.5% in patients affected by DM (Figure 1).
Discussion
Several studies have evaluated the incidence and risk factors of DM from oral cancer (3,5,8,9). However, none of these studies distinguished between patients treated with surgery and patients that did not undergo surgery. In addition, all of these studies also included patients with SCC of the oropharynx. This distinction is important because patients with SCC of oropharynx might show similar survival outcomes if treated with organ preservation protocols [radiotherapy (RT) + CT] or surgery. Moreover, they might show a higher tendency to develop DM if compared with patients with SCC affecting other locations of the oral cavity (3). In this study, we analyzed a specific population of patients affected by SCC of the oral cavity (excluding oropharynx) treated with ablative surgery ± adjuvant treatment. The incidence of DM was approximately 13.4%, which is in concordance with the 2–15% described in the literature. As shown in previous studies, the lung was the most affected organ, followed by bone and liver.
T3 and T4 stage, N2 and N3 stage, TT, perineural invasion, ECS, cervical and locoregional recurrence, clinical or pathological stage IV and contralateral neck involvement all showed a strong correlation with the risk of developing DM after primary surgery. These data are comparable with those of other studies that analyzed the risk factors associated with the appearance of DM (3,4,10). With respect to TT, it is important to emphasize that only tumors with TT >1 cm were significantly associated with DM. Importantly, no other studies have analyzed three different subgroups of TT. For instance, Aires et al. divided TT into two groups of <0.25 and >0.25 cm and they found that tumors with a thickness >0.25 cm were related to a higher risk of DM (10). However, most oral cavity tumors show a thickness greater than 0.25 cm and, thus, a more accurate estimation of the exact value of TT associated with a higher risk of developing DM could have important clinical implications. Indeed, a more precise estimation of TT value could be obtained with an accurate radiological study before surgery, which could help to identify those patients that require more aggressive clinical or surgical treatment due to the risk of cervical or distant dissemination.
van der Kamp et al., as well as several other studies, suggest that cervical metastasis is a significant risk factor for DM (3,10-12). Moreover, this risk is greater when several nodes are affected at level IV or V, or when there is contralateral neck involvement. In our series, N2 or N3 stages were independent risk factors for DM and neck involvement was the main risk factor in this study (HR =3.068; P<0.001). Cervical or locoregional recurrence was found to have the second strongest correlation with DM (HR =3.082; P<0.001).
There is controversy in the literature with regard to the relationship between perineural invasion and DM. Whilst several studies have reported a strong relationship between these two variables (perineural invasion being associated with a higher risk of developing DM), other studies have found no such association. For instance, in their study, Aires et al. reported that perineural invasion did not represent a risk factor for DM (10). In our sample, patients showing perineural invasion were found to have 1.639 times higher risk of presenting DM during follow-up. Several other studies have also reported an association between perineural invasion and poorer outcomes in terms of cervical affectation, recurrence (local, cervical and locoregional) and survival (3,10,13,14). It might therefore be assumed that perineural invasion could also play a key role in the appearance of DM.
Moreover, several studies report an association between vascular invasion and a higher risk of distant dissemination of disease (9,10,15-20). However, in the present study, vascular invasion was not found to be a relevant factor for the development of DM (P>0.005).
Interestingly, DM was diagnosed in 35% of patients analyzed in our sample (n=14) during the first 3 months after surgery [4.7% of all patients analyzed in this study (14/297)] with a chest CT or PET scan conducted before starting or after finishing adjuvant treatment. Unfortunately, it is not possible to determine if these patients had already presented distant dissemination of disease before performing the ablative surgery. However, it is it is reasonable to think that these patients presented distant metastases before undergoing primary surgery and that our staging protocol was not able to detect its. This is particularly true in the cases where there was locoregional control. As stated previously, patients presenting two or more risk factors for DM (T3 and T4 stage, N2 and N3 stage, TT, perineural invasion, ECS, cervical and locoregional recurrence, clinical or pathological stage IV) would have more than 4 times higher risk of developing distant dissemination. Several of these factors, such as TT >1 cm, T3 or T4 stage, cervical involvement, and clinical stage of disease could be accurately estimated with a careful preoperative study (Figure 2). One of the weak points of this study is represented by the fact that authors did not analyze the exact number and the location of lymph node metastases. These could represent extremely important variables for the developing of DM as suggested by Peters et al. (21). However, these factors were not analyzed in our study because neck dissection specimen was not specifically marked and oriented in some of the patients included in this study. It could be very useful to analyze these variables in future studies to facilitate a better understanding of the impact of these factors in the developing of DM. Another bias of this study could be represented by the fact that only TT, and not depth of invasion (DOI), is analyzed to predict the probability of developing DM. TT considers the depth diameter of each tumor. However, DOI is measured from the basement membrane of the epithelium from which the tumor is considered to arise, to the deepest point of invasion. Recently, several studies demonstrated that DOI has a higher prognostic value than TT (22). However, DOI was not recorded in several patients analyzed in this study. In our opinion, the analysis of this factor in future studies could be very helpful to improve our knowledge about the phenomenon of DM.
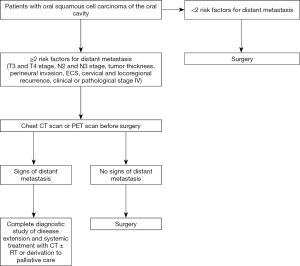
Conclusions
A chest CT scan or PET scan before surgery would be useful for patients presenting two or more risk factors for DM in order to identify patients with early distant dissemination of disease. Due to the high prevalence of lung metastasis, a chest scan would be sufficient to detect most of these cases. In this regard, a chest CT scan shows adequate sensitivity and specificity for detecting pulmonary and mediastinal metastasis. However, there is some controversy over which technique is better between CT and PET scan for the early detection of head and neck metastasis. According to Uyl-de Groot et al., PET scan would be better in terms of cost-effectiveness (23). In our opinion, the protocol reported in the present study would be useful for identifying patients with early distant dissemination, which would allow for establishing an appropriate treatment protocol for each patient. This would also reduce the morbidity associated with large and extensive surgeries.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://fomm.amegroups.org/article/view/10.21037/fomm-21-13/dss
Peer Review File: Available at https://fomm.amegroups.org/article/view/10.21037/fomm-21-13/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.org/article/view/10.21037/fomm-21-13/coif). PC serves as an unpaid editorial board member of Frontiers of Oral and Maxillofacial Medicine from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval is not required by our institution for retrospective study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- TOPAZIAN DS. Distant metastasis of oral carcinoma. Oral Surg Oral Med Oral Pathol 1961;14:705-11. [Crossref] [PubMed]
- Calhoun KH, Fulmer P, Weiss R, et al. Distant metastases from head and neck squamous cell carcinomas. Laryngoscope 1994;104:1199-205. [Crossref] [PubMed]
- Liu JC, Bhayani M, Kuchta K, et al. Patterns of distant metastasis in head and neck cancer at presentation: Implications for initial evaluation. Oral Oncol 2019;88:131-6. [Crossref] [PubMed]
- Alvi A, Johnson JT, et al. Development of distant metastasis after treatment of advanced-stage head and neck cancer. Head Neck 1997;19:500-5. [Crossref] [PubMed]
- Coca-Pelaz A, Rodrigo JP, Suárez C, et al. Clinicopathologic analysis and predictive factors for distant metastases in patients with head and neck squamous cell carcinomas. Head Neck 2012;34:771-5. [Crossref] [PubMed]
- Head and neck tumours. In: Sobin LH, Wittekind C. editors. UICC TNM classification of malignant tumours. 5th edition. New York: John Wiley & Sons, 1997:17-32.
- Moeckelmann N, Ebrahimi A, Tou YK, et al. Prognostic implications of the 8th edition American Joint Committee on Cancer (AJCC) staging system in oral cavity squamous cell carcinoma. Oral Oncol 2018;85:82-6.
- Lim JY, Lim YC, Kim SH, et al. Predictive factors of isolated distant metastasis after primary definitive surgery without systemic treatment for head and neck squamous cell carcinoma. Oral Oncol 2010;46:504-8. [Crossref] [PubMed]
- Chen TC, Hsu CW, Lou PJ, et al. The clinical predictive factors for subsequent distant metastasis in patients with locoregionally advanced oral squamous cell carcinoma. Oral Oncol 2013;49:367-73. [Crossref] [PubMed]
- Aires FT, Lin CS, Matos LL, et al. Risk Factors for Distant Metastasis in Patients with Oral Cavity Squamous Cell Carcinoma Undergoing Surgical Treatment. ORL J Otorhinolaryngol Relat Spec 2017;79:347-55. [Crossref] [PubMed]
- Leemans CR, Tiwari R, Nauta JJ, et al. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993;71:452-6. [Crossref] [PubMed]
- van der Kamp MF, Muntinghe FOW, Iepsma RS, et al. Predictors for distant metastasis in head and neck cancer, with emphasis on age. Eur Arch Otorhinolaryngol 2021;278:181-90. [Crossref] [PubMed]
- Cariati P, Cabello Serrano A, Mosalve Iglesias F, et al. What is the real prognostic value of close margins in oral oncology? Curr Probl Cancer 2019;43:100500 [Crossref] [PubMed]
- Rahima B, Shingaki S, Nagata M, et al. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:423-31. [Crossref] [PubMed]
- Adel M, Kao HK, Hsu CL, et al. Evaluation of Lymphatic and Vascular Invasion in Relation to Clinicopathological Factors and Treatment Outcome in Oral Cavity Squamous Cell Carcinoma. Medicine (Baltimore) 2015;94:e1510 [Crossref] [PubMed]
- Liao CT, Wang HM, Chang JT, et al. Analysis of risk factors for distant metastases in squamous cell carcinoma of the oral cavity. Cancer 2007;110:1501-8. [Crossref] [PubMed]
- Uchiyama Y, Sasai T, Nakatani A, et al. Distant metastasis from oral cavity-correlation between histopathology results and primary site. Oral Radiol 2021;37:167-79. [Crossref] [PubMed]
- Sekikawa S, Kawachi H, Ogane S, et al. Which Factors Affect the Long-Term Survival of Patients With Oral Squamous Cell Carcinoma With Distant Metastasis? J Oral Maxillofac Surg 2020;78:469-78. [Crossref] [PubMed]
- Tomioka H, Yamagata Y, Oikawa Y, et al. Risk factors for distant metastasis in locoregionally controlled oral squamous cell carcinoma: a retrospective study. Sci Rep 2021;11:5213. [Crossref] [PubMed]
- LU HJ. Predictive scoring systems for distant metastases in oral cavity squamous cell carcinoma. J Clin Oncol 2020; [Crossref]
- Peters TT, Senft A, Hoekstra OS, et al. Pretreatment screening on distant metastases and head and neck cancer patients: Validation of risk factors and influence on survival. Oral Oncol 2015;51:267-71. [Crossref] [PubMed]
- Piazza C, Bresciani L, Giannini L, et al. Depth of invasion for prognostic stratification in oral cavity cancer: do we need further validation? Ann Transl Med 2019;7:S84. [Crossref] [PubMed]
- Uyl-de Groot CA, Senft A, de Bree R, et al. Chest CT and whole-body 18F-FDG PET are cost-effective in screening for distant metastases in head and neck cancer patients. J Nucl Med 2010;51:176-82. [Crossref] [PubMed]
Cite this article as: Cariati P, Pampin Ozan D, Gonzalez Corcóles C, Tursun R, Arroyo Rodriguez S. Risk factors for distant metastasis in oral cancer and a strategy preoperative detection. Front Oral Maxillofac Med 2021;3:24.


