
Temporomandibular joint biosupplementation using platelet concentrates: a narrative review
Introduction
The homeostasis and movement of the temporomandibular joint (TMJ) depends on the equilibrium of its internal parts. A primary chemical or mechanical abnormality may trigger the release of inflammatory cytokines from the synovial cells or cartilage cap (1). The ensuing inflammatory cascade will lead to oxidative stress and the breakdown of joint structures. The process would begin at molecular and subclinical levels progressing to symptomatic macroscopic breakdown of joint structures (2). Examples of chemical abnormalities include excess estrone signaling through estrogen receptor-B, subsynovium seeding of bacteria or their toxins from ports of entry (gastro intestinal tract, respiratory tract, urethra), and high inflammatory processes such as in autoimmune diseases (3,4). Examples of mechanical abnormalities include joint overload through micro or macrotrauma, ligament laxity and lateral pterygoid muscle hyperactivity (5). Chemical abnormalities may weaken anatomical structures, and structural abnormalities within or around joints may promote intra-articular inflammation. Regardless of the initial trigger, intra-joint inflammation and joint degradation fuel each other in what is known as the cycle of hypoxia-reperfusion injury (6).
As a result, TMJ-internal derangement (TMJ-ID) implies an anatomical disturbance between the mandibular condyle and the articular disk (1). These are typically identified as a disc displacement with or without reduction, and with or without the presence of degenerative condylar and disc changes (7). All of which lead to a deviation from the normal biomechanics applied to the TMJ structures.
Local biosupplementation refers to the injection of an autologous product which possesses some biological activity in synovial joints, insofar as modulation of the inflammatory process, tissue repair and tissue regeneration (8). Biosupplementation, in recent years, has gained popularity instead of, and in combination with, viscosupplementation in the orthopedic and maxillofacial arenas, in the hopes that organized tissue repair or regeneration will provide long-term painless function.
The ideal therapeutic sets the stage for tissue regeneration, so that haphazard repair can be avoided. Three main categories of regenerative biologics exist (9):
- Blood or plasma concentrates which predominantly provide a source of growth factors [e.g., platelet rich plasma (PRP), platelet-rich fibrin (PRF), plasma rich in growth factors (PRGF) and concentrated growth factors (CGF)].
- Mesenchymal stem cells (MSCs) which provide pluripotential cells for cartilage, bone, and synovial regeneration. Common sources for MSCs are concentrates typically retrieved from bone marrow aspirates, adipose-derived, and peripheral blood.
- Scaffolds are structures that provide a matrix and recruitment of local MSCs for tissue regeneration.
Understanding and communicating to patients the known and potential objectives of biosupplementation as a therapeutic alternative or treatment adjunct is of paramount importance (Table 1). There are several variables that will impact these objectives: age, severity of articular disease, functional restrictions, systemic (including gastro-intestinal) health, and of course, content of the bio-product (growth factors +/− cells).
Table 1
| Hyaluronic acid | Platelet concentrates |
|---|---|
| Cushioning | Rheology |
| Lubrication | Anti-inflammatory |
| Elasticity | Anti-nociceptive effect |
| HA synthesis | |
| Chondroprotection |
PC, platelet concentrate; HA, hyaluronic acid.
As stated above, there are numerous types of biosupplements available to clinicians. Recent published studies have shown that the protocol of preparation affect the final characteristic of PCs (8). Furthermore, preclinical and clinical research in dentistry and maxillofacial surgery describe augmented healing properties (e.g., increased bone regeneration and vascular formations) by optimizing the g-force of some PCs (8). This narrative review aims to identify the latest treatment protocols implementing PCs to treat TMJ-ID alone or in combination with arthroscopy/arthrocentesis. Special focus is placed on the different plasma concentrates in terms of their content, centrifugation protocols, injection protocols, biological and clinical benefits.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://fomm.amegroups.org/article/view/10.21037/fomm-20-48/rc).
Methods
The study involves a presentation of recent data and a general discussion in the form of a narrative review of the use of platelet concentrates (PCs) as a therapeutic for TMJ-ID by direct intra-articular injection. To reduce bias in article selection, a systematic approach was used to perform the literature search following the steps described by Egger et al. (Table 2) (10).
Table 2
| Steps | General activities | Specific activities |
|---|---|---|
| I | Formation of working group | Two oral and maxillofacial surgeons with experience in blood concentrates and the treatment of TMJ-ID |
| II | Formulation of the review question | Biosupplementation as a treatment option for TMJ internal derangement. State of the art and what does the future hold? |
| III | Identification of relevant studies on PubMed, Cochrane and Key Journals | 1. Selection of keywords |
| 2. Use of Boolean operators (And; OR; NOT) | ||
| 3. Search (Table 3 search strategy) | ||
| 4. Inclusion criteria | ||
| 5. Elimination of duplicates | ||
| 6. Manual search | ||
| IV | Analyses and presentation | All data is shown in tables and graphs |
TMJ-ID, temporomandibular joint internal derangement.
Step I involved the establishment of the working team composed of two oral and maxillofacial surgeons with experience in writing literature reviews and in the treatment of TMJ-ID with PCs. In step II, both authors established the research question and defined the study variables to be extracted from the search, which were considered relevant to grasp the latest treatment options, outcomes and conclusions. The following variables were extracted: author, therapy, number of patients (NP), follow-up time (FU), baseline and final values of control and experimental groups and final conclusion from the authors. Additionally, an emphasis is placed on determining the protocol of centrifugation to obtain the PCs and the method/site of injection. Other therapies such as splint, soft diet and the use of analgesics were recorded if they were described as part of the treatment protocol. For step III, keywords relevant to the topic were used together with Boolean operators to make the search more precise and focused. The search was performed in PubMed and Cochrane and involved the evaluation of the full-text if eligible. All studies from the last 15 years related to the use of PCs as biosupplementation to treat TMJ-ID and involving human subjects were included in the study. Studies without follow-up and not reporting the protocol of centrifugation were excluded. As final step, a manual search from the references of the included articles and from key journals was conducted to extract relevant studies not found during the systematic search (Table 3). Finally, in step IV, all results were gathered in table format, analyzed and discussed. A flow chart describing the search methodology was depicted using the PRISMA template (Figure 1) (11).
Table 3
| Search strategy | Database |
|---|---|
| Systematic search | |
| Keywords: (((((((((((((((((Platelet concentrates)) OR (PRF)) OR (PRP)) OR (PRGF)) OR (Chondrocytes)) OR (Platelet rich fibrin)) OR (Platelet rich plasma)) OR (Plasma rich in growth factors)) OR (concentrated growth factor)) OR (CGF)) AND (Temporomandibular joint)) OR (TMJ)) OR (TMD) AND (dysfunction) NOT (Knee))) | PubMed, Cochrane |
| Hand search | |
| International Journal of Oral and Maxillofacial Surgery, Journal of Oral and Maxillofacial Surgery, Journal of Cranio-Maxillofacial Surgery, Oral and Maxillofacial Surgery Clinics of North America, Oral Surgery Oral Medicine Oral Pathology Oral Radiology and British Journal of Oral & Maxillofacial Surgery |
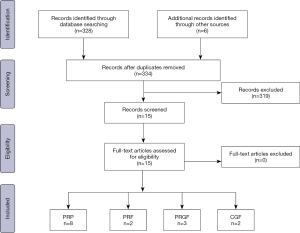
Results and discussion
Studies such as randomized controlled clinical studies, retrospective cohort studies, case series and case reports were found. The flowchart demonstrates the number of examined articles (Figure 1). In total the literature assessment led to the evaluation of 15 full-text articles, from which 11 included an experimental and a control group (Table 4). Local biosupplementation using PCs is defined as the intra or peri-articular deposition of biologics. This differs from systemic biosupplementation for which we propose as definition systemic correction of homeostatic disequilibrium, often with the aid of an anti-inflammatory nutrition (low intake of refined sugars, dairy products, gluten), and a reversal of a gut dysbiosis (12,13). Blood is an organ highly and directly influenced by systemic health, and it is therefore plausible to expect that the clinical effectiveness of an autologous blood-derived biosupplement is in turn highly dependent on systemic health (14). As such, clinical results may vary depending on the composition of biosupplements, site of injection, number of injections, disease state, age of the patient and overall health (i.e., inherent regenerative potential) of the patient (15) (Table 5).
Table 4
| Author | Therapies | NP | FU | Results | Statistically significant pain outcomes at longest FU | |
|---|---|---|---|---|---|---|
| Baseline | Final evaluation | |||||
| PRP | ||||||
| Gokçe Kutuk et al. [2019] | PRP, HA, CS | 60 | 12 | PRP: 7 (P), 53.8% (MO); HA: 8 (P), 62.5% (MO); CS: 8 (P), 83.3% (MO) | PRP: 1 (P), 0% (MO); HA: 5 (P), 50% (MO); CS: 3.4 (P), 83.3% (MO) | Single intra-articular PRP injection of 1 mL decreased TMJ palpation pain more than single HA or CS injection in TMJ-OA patients at 3 months |
| Cömert Kiliç et al. [2015] | Ar, Ar+PRP | 30 | 48 | Ar: 6.8±2.2 (P) 41.0±7.3 mm (MO); Ar+PRP: 5.7±1.3 (P) 38.7±7.8 mm (MO) | Ar: 2.4±4.8 (P), 40.2±8.9 (MO);Ar+PRP: 1.02±1.88 (P) 38.3± 8 (MO) | Ar+PRP +4 monthly PRPinjections decreased pain compared to baseline and to Ar-alone in TMJ-OA patients, at 1 year |
| Hancý et al. [2015] | PRP, Ar | 20 | 24 | PRP: 6.6±2.2 (P), 32 mm ±8.5 (MO); Ar: 6.5±2.2 (P) 30.2±9.4 (MO) | PRP: 0.07±0.2 (P) 39.7 mm±10.3 (MO); Ar: 2.7±1.4 (P) 36.3±5.5 (MO) | Single intra-articular PRPinjection of 0.6 mL for the treatment of painful reducible disc displacement decreased pain more than arthrocentesis at 6 months |
| Crockett et al. [2018] | PRP | 1 | 24 | 25 mm (MO) | PRP: 42 mm (MO) | Single PRP injection of 3 mL +3 weeks of immobilization for the treatment of painful disc displacement with reduction resolved pain and click at 6 months |
| Hegabet al. [2015] | Ar+PRP, Ar+HA | 50 | 48 | PRP: 7.3±1.1 (P) 33.8±3.0 (MO); HA: 6.9±1.2 (P) 32.4±2.7 (MO) | PRP: 0.4±0.7 (P) 41.5±2.3 (MO);HA: 1.6±1.3 (P) 39.2±2.8 (MO) | Arthrocentesis + three PRP injections of 1 mL decreased pain more than arthrocentesis +3 HA injections in TMJ-OA patients at 12 months |
| Pihut et al. [2014] | PRP | 10 | 6 | 6.5 (P) | PRP: 0.6 (P) | Single intra-articular PRP injection of 0.5 mL decreased pain compared to baseline, experienced by RDC/TMD Ia-IIIa patients at 6 weeks |
| Toameh et al. [2019] | Ar, Ar+PRP, AR+HA | 30 | 36 | Ar: 6.4 (p) 33.4 (MO); HA+Ar: 5.6 (P) 31.6 (MO); PRP+Ar: 6.10 (P) 32.3 (MO) | Ar: 2.60 (P) 42.6 (MO); HA+Ar: 1.20 (P) 54.4 (MO); PRP+Ar: 0.70 (P) 48.2 (MO) | Arthrocentesis + single PRP injection of 1 mL decreased pain more than arthrocentesis+HA or arthrocentesis alone in ADDwoR patients at 9 months |
| PRGF | ||||||
| Fernández Sanromán [2016] | Arthroscopic surgery +PRGF, arthroscopicsurgery+NS | 92 | 96 | Ar+PRGF: 7.7±1.6 (P) 26.4±6.3 (MO); A+NS: 8.1±1.9 (P) 27.2±7.6 (MO) | Ar+PRGF: 1.2±1.9 (P) 37.2±3.9 (MO); Ar+NS: 1.5±2.3 (P) 36.1±4.2 (MO) | Single 5 mL PRGF injection to IJS and SJS decrease pain compared to NS in TMJ-ID Wilkes’ IV patients, at 1 year. No difference in both groups at 2 years |
| Giacomello et al. [2015] | PRGF | 13 | 24 | 7.7±1.9 (P)30.1±4.4 (MO) | PRGF: 0.2±0.6 (P) 39.5±4.5 (MO) | Two injections of 1.5–2 mL of PRGF-Endoret to the SJS and RDT decreased pain compared to baseline represent in ADDwoR+OA patients at 6 months |
| Fernández-Ferro et al. [2017] | Arthroscopic surgery + PRGF, arthroscopic surgery +1% HA | 100 | 72 | PRGF: 8.3±0.64 (P) 27.7±4.6 (MO); HA: 8.1±0.93 (P) 27.9±5.0 (MO) | PRGF: 1.5±1.9 (MO) 37.2±4.9 (MO); HA: 2.2±1.4 (MO) 36.5±5.8 (MO) | The injection of PRGF (1 mL in IJS and 4 mL in SJS) following arthroscopic surgery reduces pain more than AS + HA in Wilkes’ IV TMJ-OA patients, at 18 months |
| PRF | ||||||
| Albilia [2020] | PRF | 37 | 48 | 5.7±2.5 (P) 33.1±6.5 (MO) | PRF: 0.8±1.2 (P) 39± 8.7 (MO) | Multiple [3–5] intra-articular liquid PRF injections of 1.5–2 mL reduce pain compared to baseline in 69% of Wilkes’ II-V TMJ-ID patients at 12 months |
| Abdelmoneim and Teama [2020] | PRF+Ar, Ar | 30 | 8 | Ar: 8±1.5 (P) 25.7±2.7 (MO); Ar+PRF: 8±1.5 (P) 25.5±3.1 (MO) | Ar: 0.5±0.5 (P)39.8±2.1 (MO); Ar+PRF: 0 (P) 45.5±3.9 (MO) | Single intra-articular PRF injection of 1.5–2 mL decreased pain significantly compared to baseline but not significant compared to arthrocentesis in ID Wilkes II-IV patients, at 8 weeks |
| CGF | ||||||
| Lin et al. [2018] | Ar, Ar+CGF | 90 | 48 | CGF: 0.95 (P), 48.3 (MO); Ar+CGF: 2.0 (P), 40.3 (MO) | CGF: 0.89 (P), 48.5 (MO); Ar+CGF: 1.47 (P), 42.0 (MO) | No significant decrease in pain in both treatment groups,compared to baseline. No significant difference in Ar+CGF compared to CGF |
| Yang et al. [2017] | CGF | 29 | 16 | 4.7±2.6 (P) 37.5±6.7 mm (MO) | CGF: 1.1±1.7 (P) 47.1±6.1 mm (MO) | Single injection of 2 mL of Liquid phase CGF in the SJS decreased TMJ pain compared to baseline in ADDwoR patients at 4 months (all patients wore a centric relation occlusal splint (CROS) at night after the injection) |
PC, platelet concentrate; TMJ-OA, temporomandibular joint-osteoarthritis; PRP, platelet rich plasma; NP, number of patients; FU, follow-up time; PRGF, plasma rich in growth factor; PRF, platelet-rich fibrin; P, pain; MO, mouth opening; HA, hyaluronic acid; CS, corticosteroid; Ar, arthrocentesis; IJS, inferior joint space; SJS, superior joint space; RDT, retrodiscal tissue.
Table 5
| Author | Protocols to obtain blood derivates (PC) (g-force, steps, additives, spin time) | Protocol of infiltration and number of infiltrations (x) |
|---|---|---|
| PRP | ||
| Gokçe Kutuk et al. [2019] | GPS III platelet separator system (Biomet Biologics, Warsaw, Poland) and was centrifuged (Model 755VES, The Drucker Company, Port Matilda, PA) in 2 sessions including soft spin (3,000 rpm, 3 minutes) allowing separation of the blood into 3 layers and then hard spin (4,000 rpm, 3 minutes) allowing formation of the PRP | 1 mL doses at a 1-month intervals under ultrasonographic guidance (3×) |
| Cömert Kiliç et al. [2015] | Sterile tubes coated with an anticoagulant (acid, citrate, and dextrose; 3.2% sodium citrate). Thesetubes were centrifuged at 1,000 rpm for 10 minutes at room temperature. The upper part of the acellular plasma was judged plasma poor in GFs and was discarded. The remaining buffy coat (PRP) was collected with a pipette. The PRP was injected into a degenerative joint without activating CaCl2 and thrombin | Arthrocentesis was performed with Ringer lactate 100 mL. Oncearthrocentesis was completed, PRP 1 mL was injected into the degenerative joint. Intra-articular PRP injections were applied after the intra-articular anesthesia on a monthly basis (4×) |
| Hancý et al. [2015] | Citrated blood samples were fractionated using centrifugation at 1,000 g for 20 min. Then the plasma of the first harvest was fractionated using centrifugation at 1,500 g for 10 min to collect the pellet. PRP was photo-activated using an AdiLight (AdiStem, Australia) for 20 min | Injection of 0.6 mL PRP into each joint. A soft diet and occlusal splint were advised for the patients after injection for one week (1×) |
| Crockett et al. [2018] | The Magellan centrifuge (Arteriocyte Medical Systems, Hopkinton, MA, USA) was used to obtain a total of 4 mL of PRP | Ultrasound guidance was used to introduce the PRP into the center of the temporal fossa and distribute the PRP at angles centrally, posteriorly, and anteriorly into the posterior ligament. A total of 3 mL of PRP was infiltrated with a 27 gauge, 1.5-inch needle. Use of splint 24 hours a day for 3 weeks (1×) |
| Hegabet al. [2015] | Closed vacuum systems and glass tubes with sodium citrate as an anticoagulant. The centrifugation parameters were 3,200 rpm and 12 minutes. After separation of the erythrocyte mass and the platelet-poor and platelet-rich plasma layers directly above the erythrocytes, the platelet-rich plasma was carefully aspirated into a separate syringe | Intra-articular injections of 1 mL PRP once per week for 3 consecutive weeks following arthrocentesis with 50 mL of Ringers lactate (3×) |
| Pihut et al. [2014] | Closed vacuum systems and glass tubes with sodium citrate as an anticoagulant. Centrifugation parameters were set to 3,200 rpm, and the centrifugation time was 12 minutes | Injections were made in the determined point with the mandible abducted. Following aspiration, 0.5 mL of plasma was injected into each temporomandibular joint (1×) |
| Rajput et al. [2020] | Glass tubes of 3.2% sodium citrate under aseptic protocol. Doublecentrifugation cycle was used for preparation of PRP. The first cycle (separating cycle) involves centrifugation of blood at 1,800 rpm for 15 min to separate erythrocytes. After centrifugation, plasma and buffy coat layer were transferred to other plain tube and centrifuged at 3,500 rpm for 10 min to concentrate platelets (concentration cycle). At the end, two layers are formed: the upper 2/3rd of PPP and lower 1/3rd of PRP, which is further separated and loaded in syringe | PRP was injected into the upper joint space (1.5 mL). Soft diet for 6 days. Occlusal splint therapy was advised after 6 months in cases of persistent pain associated with deep bites (1×) |
| Toameh et al. [2019] | 1.5 cm3 of anticoagulant (3.8% sodium citrate) was drawn into a 20-cubic centimeter syringe prior to taking blood and the inside walls were coated with the anticoagulant; 13.5 cm3 of venous blood was then drawn; afterward, the blood and the anticoagulant were mixed by slowly swinging the syringe and the gathered blood was transfused into a YcellbioTM tube (Ycellbio Medical Co. Ltd., Seoul, South Korea). The tube was then centrifuged with the following parameters: 3,400 rpm for 4 min; and RCF of 1.888 ×g. After separation, 1 mL of P-PRP, without any leukocytes, was carefully aspirated into a separate syringe; immediately before injecting it into the joint, it was activated with 0.1 mL of 10% calcium chloride | The joint was irrigated with 100 mL of Ringer’s lactate solution. 1 mL of PRP was injected into the joint before removing the needles. During the follow-up phase, we did not administer any physical therapy or splint therapy (1×) |
| PRGF | ||
| Fernández Sanromán et al. [2016] | Extraction tubes containing 3.8% sodium citrate as anticoagulant. The extracted blood was centrifuged at 580 g for 8 min at room temperature in a BTI Biotechnology Institute system centrifuge (BTI, Vitoria, Spain). Once the blood tubes were centrifuged, the plasma fractions were separated by pipetting under strictly sterile conditions. Only the 2 mL of plasma that was rich in platelets remaining above the red series was obtained; the leukocytes were avoided. Prior to infiltration, all of the 2-mL fractions were placed together in a single tube (for a total volume of 8 mL), with gentle inversion of the tube in a sterile glass container in which it was activated before infiltration via the addition of 400 μL of calcium chloride | The inferior joint space was entered under arthroscopic guidance using a spinal gauge inserted through the disc in the intermediate joint space to infiltrate 5 mL of PRGF, and the remaining PRGF was injected into the superior joint space before withdrawing the arthroscopic cannula. In the immediate post-surgical period (2 weeks), all patients received NSAIDs, consumed a soft diet, and underwent physical therapy (thermal applications and range of motion and isometric exercises) (1×) |
| Giacomello et al. [2015] | To prepare the PRGF-Endoret technology, at each treatment visit, 36 mL of peripheral blood was extracted into 4 extraction tubes containing 3.8% sodium citrate as anticoagulant. The extracted blood was centrifuged at 580 g for 8 minutes at room temperature in a Biotechnology Institute System centrifuge. Once the blood tubes were centrifuged, we proceeded to physically separate the plasma fractions by meticulous pipetting and under strictly sterile conditions. Only the 2 mL of plasma rich in platelets remaining above the red series and the “buffy coat” was pipetted, avoiding picking up the leukocytes. Before infiltration, all these 2-mL fractions were put together in a single tube (total, 8 mL), gently inverting the tube in a sterile glass container where it will be activated before infiltration, by adding 400 mL of calcium chloride | 2 injections to the suffering joint 30 days apart from each other, both in the periarticular and intra-articular area at the level of the upper articular joint space and the bilaminar (retrodiscal) zone. The volume injected was approximately 1.5 to 2 mL (2×) |
| Fernández-Ferro [2017] | Sterile tubes using 3.8% sodium citrate as an anticoagulant. The blood was then centrifuged (PRGF Centrifuge System, BTI, Vitoria, Spain) at room temperature for 8 minutes at 1,800 rpm. The platelets concentrate in the area immediately above the red cell layer, and the leukocytes are sedimented into a very thin layer immediately above the red cells. This allows a trained technician to collect the PRGF via careful pipetting to avoid contamination with leukocytes. A pipette was used to collect 100 μL of the plasma fraction. Approximately 1 cc of PRGF was obtained from each4.5 cc of blood. Activation was performed with 10% calcium chloride immediately before the injection, thereby maintaining the PRGF in liquid form, useful for intra-articular injection | Under direct visual control and by puncturing through the disc with a fine needle, PRGF was injected in liquid form into the inferior articular space (1 mL), and the rest was injected into the superior space (5 mL in total). For 15 days during the immediate postoperative period, all of the patients received treatment and recommendations nonsteroidal anti-inflammatory drugs, a bland diet (1×) |
| PRF | ||
| Albilia et al. [2020] | Sterile uncoated plastic tubes without additive (9-mL i-PRF tubes, Process for PRF, Nice, France) and immediately centrifuged. The low speed centrifugation protocol used to obtain liquid PRF was 60 g for 3 min | An auriculotemporal nerve block was performed with 0.5–1.0 cc of 3% mepivacaine. 1.5-mL of liquid PRF was deposited into the superior joint space (SJS), and 0.5 mL was distributed in the retrodiscal tissue (RT) and pericapsular area (maximum of 2 mL/joint) (2–5×) |
| Abdelmoneim and Teama [2020] | 10 mL vacutainers (plain tubes) then centrifuged for two minutes at 3,300 rpm | The affected joint was lavaged with 300 mL of saline solution concomitant with vigorous manipulation of the mandible to ensure full range of mandibular motion and then injected with 1.5 to 2 mL of injectable (flowable) PRF into the superior joint space (1×) |
| CGF | ||
| Lin et al. [2018] | Plastic whitecapped tubes (Greiner Bio-One, GmbH, Kremsmunster, Austria) without anticoagulants and reagents. These tubes were then immediately centrifuged at 2,400 to 3,000 rpm in a special machine (Medifuge; Silfradent srl, Sofia, Italy) using the following program: 30 seconds of acceleration, 2 minutes at 2,700 rpm, 4 minutes at 2,400 rpm, 4 minutes at 2,700 rpm, 3 minutes at 3000 rpm, 36 seconds of deceleration, and then stop. The CGF was then separated using a spinal needle | 2 mL of extracted CGF was injected through the G21 needle (1×) |
| Yang et al. [2017] | Plastic white cap tubes (Greiner Bio- One, GmbH, Kremsmunster, Austria) without anticoagulants and reagents. These tubes were then immediately centrifuged at 2,400 to 3,000 rpm in a special machine (Medifuge; Silfradent srl, mSofia, Italy) using a program with the following characteristics: 30 seconds acceleration, 2 min at 2,700 rpm, 4 min at 2,400 rpm, 4 min at 2,700 rpm, 3 min at 3,000 rpm, and 36 seconds deceleration and stop | 2 mL of LPCGF at point D was injected. All patients orally took 500 mg of acetaminophen once every 8 h for 3 days as necessary, with soft food for 1 week. All patients were advised to continue wearing the CROS at night after the injection, with follow-ups in the first week and every month after the injection for 6 consecutive months (1×) |
PC, platelet concentrate; PPP, platelet poor plasma; RCF, relative centrifugal force; P-PRP, pure platelet-rich plasma; CGF, concentrated growth factors; PRF, platelet-rich fibrin; LPCGF, Liquid Phase of Concentrated Growth Factors; CROS, centric relation occlusal splint.
A pre-treatment staging of the TMJ condition allows an assessment of the effectiveness of any given applied treatment. For the diagnosis, management and follow-up of patients with TMJ-ID, the Wilkes’ staging and the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) are widely accepted. The Wilkes’ staging guides the evaluation at clinical, radiological and surgical (direct view) levels and takes into account the physical state of the joint. The DC/TMD has two parts named AXIS 1 for the classification of the physical state, and AXIS 2 for the classification of the psychosocial behavior of patients (16,17). The proper imaging methods also aid in establishing an accurate diagnosis and refer to 2 dimensional images like panoramic radiography and 3 dimensional images like computer tomography, magnetic resonance and electromyography (18,19). Once a diagnosis is established, there are two main clinical outcome variables being assessed to measure improvement: pain and maximal incisal opening (MIO). Pain is measured by means of the commonly used, validated and subjective visual analogue scale (VAS) and MIO is registered by measuring the distance between the central incisors of the mandible and the maxilla (8). In this regard imaging methods, like CT and MRI, are also useful to determine improvement, especially at the bone and cartilage level (18,19). An additional method, although not described as a standard clinical assessment, is the analysis of synovial fluid aspirates. The synovial fluid of patients with TMJ-ID showed higher concentrations of pro-inflammatory cytokines and cytokine receptors, such as TNF-ɑ, IL-1β, IL-6 and active forms of MMP (20,21). Measuring markers of cytokine receptors in synovial fluid samples needs to be established as a valuable method to determine the effectiveness of biosupplementation therapy, as systemic markers of osteoarthritis (OA) disease have not been demonstrated to be reliable for monitoring cases of isolated TMJ-OA (22).
PCs
Mechanisms of action and clinical outcomes for TMJ OA
Our findings suggest that non-surgical treatment through plasma concentrates (PCs) provides an alternative therapy for painful TMJ internal derangement. The reduction of pain and dysfunction may appease surgical wait times and, in some cases, avoid the need for surgery (8).
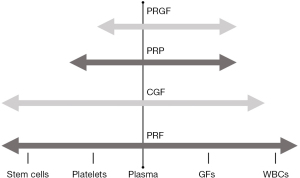
Despite the biological differences among the various PCs discussed below, there exists similarities that could explain the reported benefits from all PCs. For example, all PCs contain fibrin, fibronectin and vitronectin, which act as cell adhesion molecules for cell-cell interaction, connective tissue and epithelial migration (23). Additionally, they contain a broad range of growth factors [for example platelet-derived growth factor (PDGF), transforming growth factor beta (TFG-β), vascular endothelial growth factor (VEGF)] and various cytokines (IL-1β, IL-8, IL-4) (24-27) (Figure 2). A previous study evaluated the concentration of PDGF-BB, TFG-β, IGF-1, VEGF and bFGF in PRP, PRF and CGF. Their findings showed no statistical differences, except for bFGF, which was significantly higher in PRF and CGF compared to PRP (28). Previously, it has been hypothesized that PRF might be reducing pain as a result of its high content of the cytokine IL-4 (29). This cytokine is anti-inflammatory and modulates inflammation and pain by inhibiting MMP 1-3 and the transduction pathway from IL-1β, TNF-a and prostaglandins (8). This assumption also applies to PRGF, PRP and CGF. In a recent in vivo study, PRP, PRF and CGF were compared using a rabbit-skull defect model to evaluate bone regeneration. The results showed that all 3 protocols induced bone formation after 6 weeks without statistical differences between them (30). However, no clinical trials have been conducted up to date. Further clinical trials need to be conducted to determine if the differences in the content of each of the PCs affect the clinical outcomes, and if and how application protocols can be modified or tailored to each PC.
As demonstrated in this review, there are ample studies describing the long-term analgesic effects of all PCs (PRP, PRGF, PRF and CGF) (Table 4) (8,19,31-43), despite the absence of standardization in preparation protocols (especially for PRP) (Table 5). Although the dose (i.e., volume) per articulation and frequency of injections are not clear, it appears that multiple injections are superior to single injections, and that concomitant arthroscopic disc repositioning improve long-term analgesia and regenerative potential (8,18,40). Keeping with the apparent positive clinical outcomes with all PCs, it is reasonable that the selection of a PC should be influenced by the rationale behind the specific preparation protocol, and how this imparts its biological contents and growth factor release profile. Ease of access/cost to clinicians and patients, and the consistent reproducibility of the chosen PC content, independent of the centrifuge machine, are also reasonable factors to consider.
Although all PCs are effective for pain and dysfunction, studies with a high scientific evidence (i.e., clinical trials) comparing between PRP, PRF, PRGF and CGF to treat painful TMJ was not found, and therefore recommendations cannot be made at this point in time as to the superiority of one PC over another. All studies found reported either comparable results to control or statistically significant improvement. From the 15 studies found in the literature, PRP showed the highest accumulated number of published studies (8 articles). However, an explanation for this could be that PRP is the oldest described protocol. Furthermore, although there exists in vivo evidence of the superiority of PRP in reversing the content of inflammatory mediators compared to normal saline injection (44), there is an absence of a single human study evaluating clinical outcomes of any of the PCs, compared to normal saline (without arthrocentesis).
Some groups have demonstrated PRP’s potential to harness resident MSCs to differentiate into chondroblasts and osteoblasts in vitro and in animal studies (45). In humans, such findings have been corroborated by reparative remodeling on cone beam CT at one-year post-arthroscopic lysis and lavage in a group of TMJ-OA patients receiving multiple post-operative PRP injections at monthly intervals; these CT findings were significantly better than the arthroscopy-alone group (32,42).
Specifically for TMJ-OA, all studies included in the search (Table 4) show that PCs decrease subjective pain significantly, compared to baseline pain levels and compared to hyaluronic acid; these findings are true whether these injectables are administered as sole therapy or as adjuncts to arthroscopy/arthrocentesis. Multiple PC injections appear to offer longer lasting relief compared to single injections, but it remains to be demonstrated whether multiple PC injections are superior to arthrocentesis alone. It is clear that a single injection of PC is unlikely to provide better analgesia compared to arthrocentesis alone.
In all studies reviewed (Table 4), PCs appear to reduce subjective and objective joint noises (namely crepitus) more than hyaluronic acid or arthrocentesis, and mandibular range of motion improved similar to HA and arthroscopy/arthrocentesis.
In studies where arthroscopic surgical procedures (AS) were performed (capsulotomy, retrodiscal tissue coblation, debridement, etc.) concomitant to PC injection, it is difficult to identify whether the long-term resolution of arthralgia is attributable to the surgical procedure or to the PC, irrespective of the type of PC used. As for regenerative potential, the addition of a PC appears to offer an advantage when combined to AS, based on CBCT evidence of reparative remodeling of eroded condylar heads (32,42); such histological confirmation could not be found. Future in vivo and clinical studies are required in this regard.
No contraindications or complications were reported after the use of PCs to treat TMJ ID. However, the literature is limited in particular on the use of PC in patients with systemic arthropathies or autoimmune conditions like rheumatoid arthritis. Mainly because patients with autoimmune conditions were excluded from the studies as the treatment continues to be experimental. Patients were reported to experience temporary discomfort during the injection procedures and in the next few days (e.g., swelling and soreness) (8,33,35,43). Overall the treatment with PCs was reported to be safe.
Biological characteristics and growth factor release profile of available PCs
PRP
PRP is described to contain 3 to 5-fold the platelet concentration of normal blood or >1,000,000 platelets/mL (46). Based on the preparation protocol, PRP can be further classified as leukocyte-poor (LP-PRP) also called PRGF, or leukocyte-rich (LR-PRP). Based on subjective outcome variables, it appears that LP-PRP is superior to LR-PRP as the former activates chondrocytes, and certainly other cellular, anabolic processes (26,47). Although the best clinical results in orthopedic applications of PRP (irrespective of their leukocyte content) are found with tendinopathies, the studies reviewed in the orthopedic literature also show promising clinical results for articular applications (9). As for its GF release profile, PRP without activation releases up to 70% of its GFs within 10 minutes and nearly 100% after 1 hour (48). The activation process with calcium chloride (CaCl2) initiates the formation of fibrin and extends its GF release up to 10 days. An analysis of the growth factor release from PRP showed that after 15 minutes significantly higher levels of PDGF-AA/AB/BB, TGF-B1, VEGF, EGF and IGF-1 were released from PRP, but PRP showed lower accumulated release after 10 days compared to other PCs (49).
PRGF
PRGF-Endoret is described to possess between 2–3-fold the concentration of platelets compared to blood with almost the complete elimination of erythrocytes and leukocytes (26). The exclusion of leukocytes is supported by an in vitro study conducted by Anitua et al. The authors postulated that when comparing PRGF with or without leukocytes, the growth factor (PDGF AB, EGF, TGF-β1, IGF-1 and HGF) release profile after 8 days is not affected, but the inclusion of leukocytes increases the pro-inflammatory cytokines IL-1β and IL-16 (26). Another factor influencing the release of growth factors from PRGF is the activation method. With the aim of optimizing this protocol, an in vitro study compared the release of HGF, PDGF-BB, and IGF-1 from PRGF without undergoing activation and with activation using CaCl2 (50). The results showed that growth factor concentrations increase after undergoing activation.
Liquid platelet rich fibrin (liquid-PRF)
The characterization of this PC using immunohistochemical markers has shown that it contains platelets, white blood cells (leukocytes, T-lymphocytes, B-lymphocytes, neutrophils, granulocytes and monocytes) and GFs like VEGF, TGF-B1, EGF, PDGF-BB and MMP 9 (51). Platelet count/mm3 obtained for liquid PRF (1,434,000±75,233) was shown to be 5-fold compared to whole blood (291,000±51,575) (52). A growing body of evidence postulates PRF as a carrier of stem-like cells including haematopoietic stem cells (CD34+), endothelial progenitor cells (EPCs; CD34+/VEGR-2+/CD133+) and fibroblast-like multipotent cells (53-56). In the clinical setting, one should not confuse the solid PRF-exudate (i.e., superacute serum) with the liquid PRF matrix as these are not interchangeable in their composition. The solid PRF-exudate can be collected while pressing the solid matrices after centrifugation using a PRF-box. Liquid PRF is obtained after a protocol of centrifugation and is directly withdrawn from the tubes using a syringe. An in vitro study showed the absence of three key growth factors, namely VEGF, TGF-B1 and EGF, in the solid PRF-exudate compared to 10 days of sustained release of these in the liquid-PRF matrix. The VEGF and TGF-B1 showed a constant release while EGF peaked during the first hour with a progressive decrease up to day 10 (57).
CGF
This PC is a modification of the established PRF centrifugation protocol, however no biological differences between the two have been shown to date (58). It is described to result in a stiffer fibrin clot due to the application of higher and variable centrifugation forces with a longer processing time. The histological evaluation of CGF showed a dense fibrin matrix with platelets and leukocytes entrapped (58). Additionally, the immunohistochemical evaluation detected entrapment of circulating MSCs (CD34+) within the clot. It has been described to release growth factors for up to 7–10 days (59,60). The measurement of growth factor and cytokine levels revealed high concentrations of TGF-B1, PDGF-BB, VEGF, IL-1B and IL-6 (49).
In general, the described PCs have different cell concentrations and release kinetics of GFs, but two groups of PCs can be observed (60,61). The protocols of PRP/PRGF aim to deliver a fibrin matrix with embedded platelet and growth factors, and the protocols of PRF/CGF result in a fibrin matrix, platelets, white blood cells and stem-like cells. A trend to develop PCs with higher concentration of growth factors is evident in the literature. However, the questions of therapeutic GF concentrations, and interval of administration for a given joint to induce cartilage, bone and synovial repair and regeneration still remain to be elucidated. To date, the various release kinetics of the PCs does not seem to negatively influence the treatment outcomes of TMJ-ID. There are clinical studies where a specific GF and cytokines at different concentrations induced similar percentages of wound healing (62-65). Recombinant human PDGF-BB in gel in a concentration of 100 or 300 µg/g was applied as treatment of pressure ulcers in a randomized, double-blinded, placebo-controlled study. The topical application of both concentrations increased significantly the incidence to over 90% of healing compared to placebo without statistical differences between the concentrations (66). In a further randomized controlled study of chronic venous leg ulcers using the growth factor recombinant human granulocyte-macrophage colony-stimulating factor (rh-GM-CSF) there was a significant improvement in the treatment group compared to placebo. Fifty percent of treated patients with rh-GM-CSF healed by week 8 compared to 11% of placebo, and the use of 200 or 400 mcg showed no difference in the results (64). In an additional non-randomized study, the application of 0.5 to 1.0 mcg/cm2 of rh-GM-CSF, applied three times a week showed beneficial effects in patients with 90% of wounds showing complete healing (65). As observed, further studies are needed to understand the real benefits of higher concentrations of growth factors and whether other components, i.e., plasma, hormones, fibrin structure and the quantity of stem-like cells, play a pivotal role in their tissue regeneration capacity.
Preparation protocols of PCs
PRP
In the literature, protocols described to prepare PRP involve either a one-step or two-step centrifugation process (Figure 3). However, several differences in the protocols are observed between studies when looked closely (Table 5). In both protocols, blood is collected in tubes containing 3.2% sodium citrate, but the tube’s size and surface characteristics were not reported. The majority of protocols found in the literature are reported using revolutions per minute (RPM) only, which limits their reproducibility to the specific centrifuge machine used during the study. A one-step protocol makes use of higher centrifugation forces [3,200 rpm (35), 1,888 g (38)]. Reports of time of centrifugation varied from 3, 4 and 12 minutes. After centrifugation, blood is separated in 3 layers. The middle layer and the buffy coat are the resulting PRP. A further unstandardized procedure is the activation of the matrix. The matrix from PRP can be photo-activated or activated with 0.1 mL of 10% calcium chloride. The literature review, however, does not show consensus over this topic.
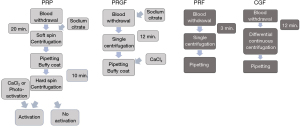
The two-step centrifugation process differs from the one-step process in that a first centrifugation, known as soft spin, is implemented using lower centrifugation forces [e.g., 1,800 rpm (37), 1,000 g (33)] and a lengthier spin time [e.g., 15 min (37), 20 min (33)]. At this point the blood is also divided in 3 layers. The upper layer is discarded and the middle layer and buffy coat are transported into a second tube without anticoagulant. A second centrifugation (hard spin) takes place using higher centrifugation forces [3,500 rpm (37), 1,500 g (33)] and reduced time [10 min (33)]. The results yield 2 layers; the upper 2/3 is discarded, as it is considered platelet poor plasma (PPP) and the remaining 1/3 is collected for treatment.
PRGF
PRGF is described as a one-step centrifugation protocol to obtain a moderate concentration of platelets and the absence of leukocytes (Figure 3) (26). Sodium citrate is used as an anticoagulant and calcium chloride, as a clot activator. Calcium chloride promotes the formation of native thrombin. Moreover, according to the developer, this procedure obviates immunological reactions and the risk of disease transmission associated with the use of exogenous bovine thrombin (26). Moreover, according to the developer, this protocol seems to have achieved standardization, as the 3 studies applying PRGF to treat TMJ-ID report the same protocol (Table 3). The reason might be that this protocol is associated with the use of only one commercial centrifuge. To prepare PRGF, blood is extracted and collected in tubes containing 3.8% sodium citrate. Tubes are centrifuged at 580 g for 8 minutes resulting in the fraction of the blood in 3 layers. The protocol specifically highlights the need for meticulous pipetting to avoid the contamination of PRGF with leukocytes. The superior layer is discarded and only the 2 mL of plasma remaining above the erythrocytes are collected. Finally, the obtained plasma is activated with 400 mL of 10% calcium chloride before infiltration (19,39,40).
PRF
PRF is defined as a second-generation PC obtained through a one-time centrifugation process, without the need of additives (i.e., sodium citrate and CaCl2) (Figure 3) (67). Depending on the centrifugation process and the tubes used, PRF can be obtained in a solid or liquid matrix. For the treatment of TMJ-ID, it is required to prepare liquid/injectable PRF. Liquid PRF was developed by reducing the centrifugation force and time, and by using uncoated plastic tubes. In the present review, differences were observed between the two PRF studies regarding the size of the tubes (8,41). This is of relevance, as the variation of the size of the tubes alters the centrifugation force. Additionally, one of the studies significantly altered the RPMs, likely altering the contents of the liquid PRF and thus reducing the reproducibility of the liquid PRF protocol (41). The original protocol for the preparation of liquid PRF is a one-time centrifugation using uncoated plastic tubes at 60 g for 3 minutes (8,51). These protocols provide approximately 2–3 mL of liquid PRF per tube of blood collected. Most recently, a new centrifugation protocol called the low speed centrifugation concept (LSCC) to obtain liquid PRF was described applying 8 minutes of centrifugation (24). The latest research producing optimized liquid PRF is 44 g for 8 minutes (24). Through a longer spin time, it is possible to obtain up to 3–4 mL of liquid PRF from a 10 mL blood sample. The availability to work with more liquid PRF, that undergoes slow and natural coagulation, with the same amount of blood, makes it clinically attractive for procedures like biomaterial handling, wound coverage, bone regeneration, or intra-articular injection of larger joints. It is critical to understand that although lower RCFs yield smaller PC volumes, these PCs are maximized insofar as their concentration of growth factors, cytokines and cellular content, and thus their therapeutic/regenerative potential (68-71) (Figure 4). It has been demonstrated that higher centrifugation protocols (higher g-force) reduce the cell content and the concentration of growth factors in PRF (24). Furthermore, horizontal centrifugation was introduced as an apparent improvement to fixed-angle centrifugation (72). According to Miron et al., on a fixed-angel centrifuge, cells are pressed against the wall of the tube while spinning, causing shear stress on cells and possible cell rupture (73). Horizontal centrifugation allows for free cell movement and a more uniform separation of the cells in the tube (72). By comparing 24 protocols of horizontal centrifugation of platelet-rich fibrin (H-PRF), a recent study determined that the optimal protocols for solid and liquid H-PRF are in the range of 400–700 g for 8 minutes and 200–400 g for 5 minutes respectively (73). Further studies are necessary to determine whether this represents a real clinical improvement compared to other PCs. To date, there are no studies reporting the use of horizontal centrifugation to treat TMJ-ID.

CGF
Its name originates from the statement of the author that CGF contains a higher concentration of growth factors compared to other PCs. CGF is produced without anticoagulant by variable and continuous centrifugation ranging from 2,400 to 2,700 rpm (74).
This protocol is likely standardized as it appears to be associated with a commercial centrifuge machine. The protocol of preparation is known as differential continuous centrifugation. The authors postulate that due to the alternating centrifugation forces, cells collide with the tube wall causing the rupture of platelets resulting in a higher release of growth factors (74). For a liquid phase CGF, the protocol is described as follows: blood is collected in plastic tubes without anticoagulants and immediately centrifuged using 30 seconds of acceleration, 2 minutes at 2,700 rpm, followed by 4 minutes at 2,400 rpm, followed by 4 minutes at 2,700 rpm, followed by 3 minutes at 3,000 rpm, and finally 36 seconds of deceleration to a full stop (42,43). Once finalized, the middle layer of the tube is retrieved and injected in the joint.
Challenges in clinical applications of PCs
Absence of standardization
In the literature, varying centrifugation protocols to obtain PCs are described. However, recently published studies have shown that the characteristics of the centrifuge machine and the characteristics of a tube have an effect over the resulting PC. A mnemonic developed by Herrera-Vizcaino C. to correctly report PCs is the acronym AR2T3 (manuscript in preparation). This acronym includes all principles affecting the final content within the PCs. According to AR2T3, a protocol reporting on a PC should include the (A) angulation of the tube within the centrifuge, (R) the radius maximum (r-max), (R) the relative centrifugation force (g-force), (T) the time (duration) of centrifugation, (T) the characteristics of the tube’s surface and (T) the tube’s size. The aforementioned principles could be embodied within research articles using the following template: “PRF was centrifuged using a table-top centrifuge (name of the centrifuge machine; angle X°, X cm radius-max) following a standardized protocol described by X etc. (X rpm, X g, X min). Blood was withdrawn according to the best practices in phlebotomy into X-mL sterile (characteristic of the tube; plastic, glass or titanium) tubes (name of the manufacture). This protocol was reported following the AR2T3 acronym.”
In the meantime, clinicians are encouraged to inquire about, or calculate, the relative centrifugal force (RCF) or g-force exerted on the harvested biologic by using the following formula: g-force (RCF) = (RPM)2 ×1.118×10−5× r, where r is the maximum radius from the tube to the center of the centrifuge rotor. The purpose of which is to keep within an optimal g-force range for the ideal balance of growth factors and cellular contents, for either liquid or solid PC preparations (75).
Red blood cell (RBC) hemolysis
Manipulation of whole blood specimens with shear forces beyond the shear strength of RBCs can cause eryptosis within the biologic (76). RBC hemolysis or eryptosis releases excessive amounts of hemoglobin (Hb) and its toxic byproducts heme and iron, rapidly overwhelming the body’s innate neutralizing capabilities (76). Also devastating is plasma-free Hb (PFH) causing physiological effects such as endothelial damage and dysfunction, depletion of nitric oxide and vasoconstriction, cytotoxicity and release of reactive oxygen species and pro-inflammatory infiltrates, and suppressed MSC recruitment (76). RBCs are also a reservoir of macrophage inhibitory factor (MIF), greater than that found in platelets and leukocytes (76); release of potent MIF is a well-documented cause of inflammatory cytokine chemotaxis in painful OA (77,78).
The manipulation of blood or bone marrow aspirates requires delicate handling in the harvesting technique, centrifugation protocol (namely g-force), and injection technique, so as to reduce RBC trauma, especially since the body’s natural circulating scavenging systems for Hb byproducts and PFH are entirely bypassed, in the biologic treatment vial, syringe and in the target tissue’s microenvironment (76). Careful evaluation of the state of RBCs post processing is paramount in selecting the ideal biologic. Several laboratory investigations have confirmed that centrifugal forces in the range of 500–1,500 g for 10 minutes impart no hemolytic damage to blood samples; however repeat centrifugation cycles within such a g-force range could raise levels of PFH within the supernatant (79).
Effects of suboptimal systemic health in patient samples
Knowledge of this potential concern is inexistent insofar as its effect on the composition of PCs. That being said, there is mounting evidence that gut dysbiosis fuels systemic, and indeed, articular inflammation. As such, probiotic supplementation and nutritional corrections reverse dysbiosis to a normal gut microbiome (80), which in turn optimize the immune system.
Absence of standardization in injection technique/site and in combination therapies
Most reports included herein describe the deposition of PCs in the superior joint space (Figure 5). Although from a technical standpoint, this seems like an appropriate space to introduce the maximum volume for the TMJ, there are studies that suggest treatment of the inferior joint space as highly beneficial (40,81). The TMJ’s inferior joint space can be accessed by arthroscopic guidance or by experienced injectors (40). It is logical to believe that treatment of this space may be very beneficial insofar as improving joint rheology and the regenerative potential of PCs by recruitment of fibrochondrocyte stem cells found in the superficial layer of the condylar cartilage (82).
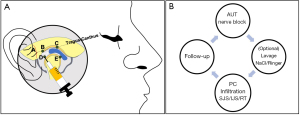
Whether PCs offer superior outcomes as an adjunctive therapy to arthrocentesis or can some PCs induce a natural joint lavage, in part by its vascular recruitment as some have suggested (8), requires further investigation. Our findings showed positive outcomes in all studies independent of whether lavage was performed. Although it has been described that the optimal volume to remove inflammatory mediators responsible for pain lays between 200 and 300 mL (83), authors describe using Ringer’s lactate or 0.9% NaCl in a range from 50 to 100 mL (Table 5). A study with 17 patients with TMJ-ID were treated with 50, 100, 200, 300 or 400 mL. The results showed that the perfusion with over 200 mL causes a statistically significant reduction in the concentrations of the pro—inflammatory mediators Bradykinin, IL-6 and total protein, making them undetectable in synovial fluid (83). In light of this information, it would be interesting to compare outcomes of the cumulative effect of multiple PC injections at 2–4-week intervals to arthrocentesis alone, to determine if PCs can provide a physiologic lavage by progressively modifying the TMJ’s microenvironment. Noteworthy, arthroscopic surgery involving capsulotomy, debridement and retrodiscal coagulation, improving functional TMJ anatomy, appears to optimize long-term outcomes when followed by a PC, compared to arthrocentesis (Table 4).
MSCs
Although this review focuses on PCs, a brief discussion on MSCs as they relate to articular disease is warranted. MSCs make up 0.001% to 0.01% of mononuclear cells in bone marrow aspirates (84); they possess significant anabolic properties (and anti-catabolic properties via secretion of IL-1 receptor antagonist) on target tissues, provide a significant source of GFs and tissue-specific cells capable of matrix formation (i.e., blast cells) (9).
Stem cell therapy is a rapidly expanding field in regenerative medicine and its application in orthopedics is FDA approved. MSCs possess the multipotency to differentiate into osteoblasts, chondroblasts and synoviocytes. These are typically retrieved from bone marrow aspirates (BM-MSCs) and they can be combined with PCs to improve their osteoblastic/chondroblastic differentiation, as recently shown (85). Furthermore, in a recent animal study, PRP was used as a carrier of BM-MSCs on the surface of a damaged cartilage murine model. The histological results showed a complete repair of the articular surface with the presence of cartilage-like tissue and subchondral bone filling compared to the control group, which showed no signs of repair (86). MSCs can also be found in adipose tissue and blood; furthermore, these can be harnessed locally in an injured tissue. The latter two sources of MSCs (blood and tissue-resident) offer procedural simplicity for the ambulatory setting.
In 2016, it has been demonstrated for the first time that the superficial zone of the adult TMJ is a niche of fibrocartilage stem cells with both chondrogenic and osteogenic potential. Such is not the case in articulations covered with hyaline cartilage, and thus fibrocartilage stem cells can be tested for repair of injured hyaline cartilage, as hyaline cartilage is naturally repaired with fibrocartilage. Interestingly, severely arthritic joints exhibit capillary tidemarks that provide access to chondro-progenitor cells into injured joints (82). It is thus reasonable to consider PCs as a means to recruit and activate MSCs with chondrogenic/osteogenic potential, and thus bypass the risks and concerns of stem cell therapy (85,87).
MSCs possess immunomodulatory properties in acute inflammatory settings, but this should not be the main reason for their application in painful arthritic joints, as similar effects have been demonstrated in vivo and in vitro with the various PCs (8). Furthermore, there is evidence that MSCs can be pro-inflammatory in a chronic inflammatory environment, which is often the case in chronic conditions such as painful OA (9).
Conclusions
PCs have different cell concentrations and release kinetics of GFs. The protocols of PRP/PRGF aim to deliver a fibrin matrix with embedded platelet and growth factors, and the protocols of PRF/CGF result in a fibrin matrix, platelets, white blood cells and stem-like cells. Nevertheless, all studies reported PCs as a beneficial biosupplementation treatment for painful TMJ-ID. This review is limited in that it cannot yet classify a single PC as the type with the strongest evidence. However, all studies included in the review showed that PCs decrease subjective pain significantly and these findings are true whether these injectables are administered as sole therapy or as adjuncts to arthroscopy/arthrocentesis. Furthermore, multiple PC injections offer longer lasting relief compared to single injections. Despite the biological differences among the various PCs, there exists similarities that could explain the reported benefits. There are other aspects that need further consideration in future research; like the effects of suboptimal systemic health in patient samples when collecting blood and the release of toxic bioproduct from RBC hemolysis after the use of excessive centrifugation forces. Furthermore, standardization of centrifugation and injection protocols should be a main concern in order to improve reproducibility and homogeneity of results. The authors recommend that clinicians implement the acronym AR2T3 to correctly report PC preparations in future studies. Altogether, our findings suggest that non-surgical treatment through biosupplementation provides an effective therapy for TMJ-ID.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Stephen Feinberg and Louis Mercuri) for the series “Temporomandibular Joint Disorders Diagnosis and Management – What Does the Future Hold?” published in Frontiers of Oral and Maxillofacial Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://fomm.amegroups.org/article/view/10.21037/fomm-20-48/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.org/article/view/10.21037/fomm-20-48/coif). The series “Temporomandibular Joint Disorders Diagnosis and Management – What Does the Future Hold?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nitzan DW. Intraarticular pressure in the functioning human temporomandibular joint and its alteration by uniform elevation of the occlusal plane. J Oral Maxillofac Surg 1994;52:671-9. [Crossref] [PubMed]
- Murphy MK, MacBarb RF, Wong ME, et al. Temporomandibular Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. Int J Oral Maxillofac Implants 2013;28:e393-414. [Crossref] [PubMed]
- Park Y, Chen S, Ahmad N, et al. Estrogen Selectively Enhances TMJ Disc but Not Knee Meniscus Matrix Loss. J Dent Res 2019;98:1532-8. [Crossref] [PubMed]
- GH. K. Reactive arthritis: a paradigm for inflammatory arthritis. Clin Exp Rheumatol 1993;11:29-36.
- Nitzan DW, Etsion I. Adhesive force: the underlying cause of the disc anchorage to the fossa and/or eminence in the temporomandibular joint--a new concept. Int J Oral Maxillofac Surg 2002;31:94-9. [Crossref] [PubMed]
- Israel HA, Langevin CJ, Singer MD, et al. The Relationship Between Temporomandibular Joint Synovitis and Adhesions: Pathogenic Mechanisms and Clinical Implications for Surgical Management. J Oral Maxillofac Surg 2006;64:1066-74. [Crossref] [PubMed]
- Holmlund AB, Axelsson S, Gynther GW. A comparison of discectomy and arthroscopic lysis and lavage for the treatment of chronic closed lock of the temporomandibular joint: A randomized outcome study. J Oral Maxillofac Surg 2001;59:972-7. [Crossref] [PubMed]
- Albilia J, Herrere-Vizcaíno C, Weisleder H, et al. Liquid platelet-rich fibrin injections as a treatment adjunct for painful temporomandibular joints: preliminary results. Cranio 2020;38:292-304. [Crossref] [PubMed]
- Chahla J, Cinque M, LaPrade RF, et al. Overview of Orthobiology and Biomechanics. In: Bio-orthopaedics 2017:25-40.
- Egger M,Smith GD,Altman D. Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Books, editor. NJ, USA, John Wiley & Sons; 2001.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Drago L, Panelli S, Bandi C, et al. What Pediatricians Should Know Before Studying Gut Microbiota. J Clin Med 2019;8:1206. [Crossref] [PubMed]
- Messina OD, Vidal Wilman M, Vidal Neira LF. Nutrition, osteoarthritis and cartilage metabolism. Aging Clin Exp Res 2019;31:807-13. [Crossref] [PubMed]
- Mussano F, Genova T, Munaron L, et al. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016;27:467-71. [Crossref] [PubMed]
- Miron RJ, Dham A, Dham U, et al. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig 2019;23:2179-85. [Crossref] [PubMed]
- Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28:6-27. [Crossref] [PubMed]
- Wilkes CH. Internal Derangements of the Temporomandibular Joint. Arch Otolaryngol Head Neck Surg 1989;115:469-77. [Crossref] [PubMed]
- Cömert Kiliç S, Güngörmüş M, Sümbüllü MA. Is Arthrocentesis Plus Platelet-Rich Plasma Superior to Arthrocentesis Alone in the Treatment of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial. J Oral Maxillofac Surg 2015;73:1473-83. [Crossref] [PubMed]
- Fernández Sanromán J, Fernández Ferro M, Costas López A, et al. Does injection of plasma rich in growth factors after temporomandibular joint arthroscopy improve outcomes in patients with Wilkes stage IV internal derangement? A randomized prospective clinical study. Int J Oral Maxillofac Surg 2016;45:828-35. [Crossref] [PubMed]
- Kaneyama K, Segami N, Nishimura M, et al. Importance of proinflammatory cytokines in synovial fluid from 121 joints with temporomandibular disorders. Br J Oral Maxillofac Surg 2002;40:418-23. [Crossref] [PubMed]
- Kaneyama K, Segami N, Sun W, et al. Analysis of tumor necrosis factor-alpha, interleukin-6, interleukin-1beta, soluble tumor necrosis factor receptors I and II, interleukin-6 soluble receptor, interleukin-1 soluble receptor type II, interleukin-1 receptor antagonist, and protein in the synovial fluid of patients with temporomandibular joint disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:276-84. [Crossref] [PubMed]
- Wadhwa S, Kapila S. TMJ Disorders: Future Innovations in Diagnostics and Therapeutics. J Dent Educ 2008;72:930-47. [Crossref] [PubMed]
- Marx RE. Platelet-Rich Plasma: Evidence to Support Its Use. J Oral Maxillofac Surg 2004;62:489-96. [Crossref] [PubMed]
- Choukroun J, Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg 2018;44:87-95. [Crossref] [PubMed]
- Zotti F, Albanese M, Rodella LF, et al. Platelet-rich plasma in treatment of temporomandibular joint dysfunctions: Narrative review. Int J Mol Sci 2019;20:277. [Crossref] [PubMed]
- Anitua E, Zalduendo MM, Prado R, et al. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: Evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A 2015;103:1011-20. [Crossref] [PubMed]
- Yu M, Wang X, Liu Y, et al. Cytokine release kinetics of concentrated growth factors in different scaffolds. Clin Oral Investig 2019;23:1663-71. [Crossref] [PubMed]
- Qiao J, An N, Ouyang X. Quantification of growth factors in different platelet concentrates. Platelets 2017;28:774-8. [Crossref] [PubMed]
- Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e51-5. [Crossref] [PubMed]
- Kim TH, Kim SH, Sádor GK, et al. Comparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF) in rabbit-skull defect healing. Arch Oral Biol 2014;59:550-8. [Crossref] [PubMed]
- Gokçe Kutuk S, Gökçe G, Arslan M, et al. Clinical and radiological comparison of effects of platelet-rich plasma, hyaluronic acid, and corticosteroid injections on temporomandibular joint osteoarthritis. J Craniofac Surg 2019;30:1144-8. [Crossref] [PubMed]
- Cömert Kiliç S, Kiliç N, Sümbüllü MA. Temporomandibular joint osteoarthritis: Cone beam computed tomography findings, clinical features, and correlations. Int J Oral Maxillofac Surg 2015;44:1268-74. [Crossref] [PubMed]
- Hancı M, Karamese M, Tosun Z, et al. Intra-articular platelet-rich plasma injection for the treatment of temporomandibular disorders and a comparison with arthrocentesis. J Craniomaxillofac Surg 2015;43:162-6. [Crossref] [PubMed]
- Crockett KL, Bourassa R, Friesen T. Anterior disc derangement with reduction of the temporomandibular joint: A case report. J Med Case Rep 2018;12:148. [Crossref] [PubMed]
- Hegab AF, Ali HE, Elmasry M, et al. Platelet-rich plasma injection as an effective treatment for temporomandibular joint osteoarthritis. J Oral Maxillofac Surg 2015;73:1706-13. [Crossref] [PubMed]
- Pihut M, Szuta M, Ferendiuk E, et al. Evaluation of Pain Regression in Patients with Temporomandibular Dysfunction Treated by Intra-Articular Platelet-Rich Plasma Injections: A Preliminary Report. Biomed Res Int 2014;2014:132369 [Crossref] [PubMed]
- Rajput A, Bansal V, Dubey P, et al. A Comparative Analysis of Intra-articular Injection of Platelet-Rich Plasma and Arthrocentesis in Temporomandibular Joint Disorders. J Maxillofac Oral Surg 2020; [Crossref]
- Toameh MH, Alkhouri I, Karman MA. Management of patients with disk displacement without reduction of the temporomandibular joint by arthrocentesis alone, plus hyaluronic acid or plus platelet-rich plasma. Dent Med Probl 2019;56:265-72. [Crossref] [PubMed]
- Giacomello M, Giacomello A, Mortellaro C, et al. Temporomandibular joint disorders treated with articular injection: the effectiveness of plasma rich in growth factors-Endoret. J Craniofac Surg 2015;26:709-13. [Crossref] [PubMed]
- Fernández-Ferro M, Fernández-Sanromán J, Blanco-Carrión A, et al. Comparison of intra-articular injection of plasma rich in growth factors versus hyaluronic acid following arthroscopy in the treatment of temporomandibular dysfunction: A randomised prospective study. J Craniomaxillofac Surg 2017;45:449-54. [Crossref] [PubMed]
- Abdelmoneim H, Teama U. Evaluation of Injectable platelet rich fibrin for the management of Tempromandibular joint internal derangement. (clinical evaluation). Egypt Dent J 2020;66:883-91. [Crossref]
- Lin SL, Tsai CC, Wu SL, et al. Effect of arthrocentesis plus platelet-rich plasma and platelet-rich plasma alone in the treatment of temporomandibular joint osteoarthritis: A retrospective matched cohort study (A STROBE-compliant article). Medicine (Baltimore) 2018;97:e0477 [Crossref] [PubMed]
- Yang JW, Huang YC, Wu SL, et al. Clinical efficacy of a centric relation occlusal splint and intra-articular liquid phase concentrated growth factor injection for the treatment of temporomandibular disorders. Medicine (Baltimore) 2017;96:e6302 [Crossref] [PubMed]
- Lippross S, Moeller B, Haas H, et al. Intraarticular injection of platelet-rich plasma reduces inflammation in a pig model of rheumatoid arthritis of the knee joint. Arthritis Rheum 2011;63:3344-53. [Crossref] [PubMed]
- Ramezanifard R, Kabiri M, Ahvaz HH, et al. Effects of Platelet Rich Plasma and Chondrocyte Co-Culture on Msc Chondrogenesis, Hypertrophy and. J Exp Clin Sci 2017;1031-45.
- Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: A randomized, single-blind, parallel-group trial. Am J Sports Med 2013;41:2240-8. [Crossref] [PubMed]
- Cavallo C, Filardo G, Mariani E, et al. Comparison of platelet-rich plasma formulations for cartilage healing: An in vitro study. J Bone Joint Surg Am 2014;96:423-9. [Crossref] [PubMed]
- Sánchez-González DJ, Méndez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: Key for regeneration. Int J Pept 2012;2012:532519 [Crossref] [PubMed]
- Masuki H, Okudera T, Watanebe T, et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int J Implant Dent 2016;2:19. [Crossref] [PubMed]
- Kikuchi N, Yoshioka T, Taniguchi Y, et al. Optimization of leukocyte-poor platelet-rich plasma preparation: a validation study of leukocyte-poor platelet-rich plasma obtained using different preparer, storage, and activation methods. J Exp Orthop 2019;6:24. [Crossref] [PubMed]
- Wend S, Kubesch A, Orlowska A, et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J Mater Sci Mater Med 2017;28:188. [Crossref] [PubMed]
- Karde PA, Sethi KS, Mahale SA, et al. Comparative evaluation of platelet count and antimicrobial efficacy of injectable platelet-rich fibrin with other platelet concentrates: An in vitro study. J Indian Soc Periodontol 2017;21:97-101. [Crossref] [PubMed]
- Di Liddo R, Bertalot T, Borean A, et al. Leucocyte and Platelet-rich Fibrin: a carrier of autologous multipotent cells for regenerative medicine. J Cell Mol Med 2018;22:1840-54. [Crossref] [PubMed]
- Kucia MJ, Wysoczynski M, Wu W, et al. Evidence That Very Small Embryonic-Like Stem Cells Are Mobilized into Peripheral Blood. Stem Cells 2008;26:2083-92. [Crossref] [PubMed]
- Caloprisco G, Borean A, De Angeli S, et al. New method to produce hemocomponents for regenerative use from peripheral blood: Integration among platelet growth factors monocytes and stem cells. Transfus Apher Sci 2010;42:117-24. [Crossref] [PubMed]
- Barbon S, Stocco E, Macchi V, et al. Platelet-rich fibrin scaffolds for cartilage and tendon regenerative medicine: From bench to bedside. Int J Mol Sci 2019;20:1701. [Crossref] [PubMed]
- Al-Maawi S, Herrera-Vizcaino C, Dohle E, et al. Homogeneous pressure influences the growth factor release profiles in solid platelet-rich fibrin matrices and enhances vascular endothelial growth factor release in the solid platelet-rich fibrin plugs. Int J Growth Factors Stem Cells Dent 2018;1:8-16.
- Bernardi S, Mummolo S, Tecco S, et al. Histological Characterization of Sacco’s Concentrated Growth Factors Membrane. Int J Morphol 2017;35:114-9. [Crossref]
- Kobayashi E, Flückiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 2016;20:2353-60. [Crossref] [PubMed]
- Wang L, Wan M, Li Z, et al. A comparative study of the effects of concentrated growth factors in two different forms on osteogenesis in vitro. Mol Med Rep 2019;20:1039-48. [Crossref] [PubMed]
- Miron RJ, Fujioka-Kobayashi M, Hernandez M, et al. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig 2017;21:2619-27. [Crossref] [PubMed]
- Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 2014;22:569-78. [Crossref] [PubMed]
- Da Costa RM, Ribeiro Jesus FM, Aniceto C, et al. Randomized, double-blind, placebo-controlled, dose- ranging study of granulocyte-macrophage colony stimulating factor in patients with chronic venous leg ulcers. Wound Repair Regen 1999;7:17-25. [Crossref] [PubMed]
- Marques da Costa R, Jesus FM, Aniceto C, et al. Double-blind randomized placebo-controlled trial of the use of granulocyte-macrophage colony-stimulating factor in chronic leg ulcers. Am J Surg 1997;173:165-8. [Crossref] [PubMed]
- Jaschke E, Zabernigg A, Gattringer C. Recombinant human granulocyte-macrophage colony-stimulating factor applied locally in low doses enhances healing and prevents recurrence of chronic venous ulcers. Int J Dermatol 1999;38:380-6. [Crossref] [PubMed]
- Rees RS, Robson MC, Smiell JM, et al. Becaplermin gel in the treatment of pressure ulcers: A phase II randomized, double-blind, placebo-controlled study. Wound Repair Regen 1999;7:141-7. [Crossref] [PubMed]
- Ghanaati S, Herrera-Vizcaino C, Al-Maawi S, et al. Fifteen years of platelet rich fibrin in dentistry and oromaxillofacial surgery: How high is the level of scientific evidence? J Oral Implantol 2018;44:471-92. [Crossref] [PubMed]
- El Bagdadi K, Kubesch A, Yu X, et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept). Eur J Trauma Emerg Surg 2019;45:467-79. [Crossref] [PubMed]
- Kubesch A, Barbeck M, Al-Maawi S, et al. A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets 2019;30:329-40. [Crossref] [PubMed]
- Herrera-Vizcaíno C, Dohle E, Al-Maawi S, et al. Platelet-rich fibrin secretome induces three dimensional angiogenic activation in vitro. Eur Cell Mater 2019;37:250-64. [Crossref] [PubMed]
- Verboket R, Herrera-Vizcaíno C, Thorwart K, et al. Influence of concentration and preparation of platelet rich fibrin on human bone marrow mononuclear cells (in vitro). Platelets 2019;30:861-70. [Crossref] [PubMed]
- Fujioka-Kobayashi M, Kono M, Katagiri H, et al. Histological comparison of Platelet rich fibrin clots prepared by fixed-angle versus horizontal centrifugation. Platelets 2021;32:413-9. [Crossref] [PubMed]
- Miron RJ, Chai J, Fujioka-Kobayashi M, et al. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health 2020;20:310. [Crossref] [PubMed]
- Hong S, Chen W, Jiang B. A Comparative Evaluation of Concentrated Growth Factor and Platelet-rich Fibrin on the Proliferation, Migration, and Differentiation of Human Stem Cells of the Apical Papilla. J Endod 2018;44:977-83. [Crossref] [PubMed]
- Ghanaati S, Mourão CF, Adam EH, et al. The Role of Centrifugation Process in the Preparation of Therapeutic Blood Concentrates: Standardization of the Protocols to Improve Reproducibility. Int J Growth Factors Stem Cells Dent 2019;1:32-7. [Crossref]
- Everts PA, Malanga GA, Paul RV, et al. Assessing clinical implications and perspectives of the pathophysiological effects of erythrocytes and plasma free hemoglobin in autologous biologics for use in musculoskeletal regenerative medicine therapies. A review. Regen Ther 2019;11:56-64. [Crossref] [PubMed]
- Zhang PL, Liu J, Xu L, et al. Synovial fluid macrophage migration inhibitory factor levels correlate with severity of self- reported pain in knee osteoarthritis patients. Med Sci Monit 2016;22:2182-6. [Crossref] [PubMed]
- Liu M, Hu C. Association of MIF in serum and synovial fluid with severity of knee osteoarthritis. Clin Biochem 2012;45:737-9. [Crossref] [PubMed]
- Mancuso JE, Jayaraman A, Ristenpart WD. Centrifugation-induced release of ATP from red blood cells. PLoS One 2018;13:e0203270 [Crossref] [PubMed]
- Drago L. Prevotella Copri and Microbiota in Rheumatoid Arthritis: Fully Convincing Evidence? J Clin Med 2019;8:1837. [Crossref] [PubMed]
- Li C, Zhang Y, Lv J, et al. Inferior or double joint spaces injection versus superior joint space injection for temporomandibular disorders: A systematic review and meta-analysis. J Oral Maxillofac Surg 2012;70:37-44. [Crossref] [PubMed]
- Embree MC, Chen M, Pylawka S, et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat Commun 2016;7:13073. [Crossref] [PubMed]
- Kaneyama K, Segami N, Nishimura M, et al. The ideal lavage volume for removing bradykinin, interleukin-6, and protein from the temporomandibular joint by arthrocentesis. J Oral Maxillofac Surg 2004;62:657-61. [Crossref] [PubMed]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-7. [Crossref] [PubMed]
- Simon M, Major B, Vácz G, et al. The effects of hyperacute serum on the elements of the human subchondral bone marrow niche. Stem Cells Int 2018;2018:4854619 [Crossref] [PubMed]
- Gomez M, Wittig O, Diaz-Solano D, et al. Mesenchymal Stromal Cell Transplantation Induces Regeneration of Large and Full-Thickness Cartilage Defect of the Temporomandibular Joint. Cartilage 2020; [Crossref] [PubMed]
- Kuten O, Simon M, Hornyák I, et al. The Effects of Hyperacute Serum on Adipogenesis and Cell Proliferation of Mesenchymal Stromal Cells. Tissue Eng Part A 2018;24:1011-21. [Crossref] [PubMed]
Cite this article as: Herrera-Vizcaino C, Albilia JB. Temporomandibular joint biosupplementation using platelet concentrates: a narrative review. Front Oral Maxillofac Med 2021;3:38.

