
Antimicrobial adjuncts in the management of periodontal and peri-implant diseases and conditions: a narrative review
Introduction
Periodontal diseases
Gingivitis is defined as “an inflammatory lesion resulting from interactions between the dental plaque biofilm and the host’s immune-inflammatory response”. This inflammatory lesion of gingivitis remains contained within the gingiva and does not extend to the periodontal attachment (cementum, periodontal ligament and alveolar bone) (1). Such inflammation associated with gingivitis is reversible by reducing levels of dental plaque.
Periodontitis is a chronic multifactorial inflammatory disease (2). This chronic inflammation is a serious infection due to its prevalence and if left untreated, may lead to tooth loss and other possible infective systemic consequences (2). The progressive disease of periodontitis is associated with dysbiotic plaque biofilms and is characterized by destruction of the tooth-supporting apparatus. While common, this life-long disease can generally be controlled. Most patients with periodontitis manifest the adult chronic form of this disease, according to the 1999 Armitage classification (3). However, the World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions (4-7) defined forms of periodontal diseases, such as periodontitis, periodontitis as a manifestation of systemic diseases and necrotizing periodontal diseases. Other periodontal conditions include abscesses of the periodontium, endodontic-periodontal lesions, developmental or acquired deformities and conditions. Therefore, management differs depending on the specific type of periodontal disease. The 2017 World Workshop’s new classification of periodontal diseases aims to clearly identify clinical entities and accurately link diagnosis with treatment (8). This is a major change from the previous classification system published in 1999 that recognized different forms of periodontitis (chronic, aggressive, manifestation of systemic diseases) (3,8). The terms “chronic” and “aggressive” are no longer used because the distinction between them cannot be currently justified as their etiology is the same (8). A patient with a periodontitis diagnosis needs to be assigned a stage and grade of periodontitis (2,4,8,9). Since this narrative review cites both studies prior to 2017 (using the previous periodontitis classification) and since 2017 (using the new classification of periodontitis), references of both systems are outlined. Summary of the staging and grading of periodontitis according to the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions is briefly outlined in Table 1.
Table 1
| Periodontitis classification | Description of periodontitis | Diagnostic criteria |
|---|---|---|
| Stage 1 | Initial | PD ≤4 mm; CAL =1–2 mm; RBL ≤15% |
| Stage 2 | Moderate | PD ≤5 mm; CAL =3–4 mm; RBL =15–33% |
| Stage 3 | Severe | PD ≥6 mm; CAL ≥5 mm; RBL >33%; VBL ≥3 mm; furcation class 2 or 3; moderate ridge defect |
| Stage 4 | Advanced | Stage 3 criteria AND ≥5 teeth lost due to periodontitis AND need for complex rehabilitation |
| Grade A | Slow rate of Periodontitis progression | No CAL or RBL over 5 years; nonsmoker; normoglycemic |
| Grade B | Moderate rate of Periodontitis progression | CAL or RBL <2 mm over 5 years; tissue destruction is as expected given the level of biofilm deposits |
| Grade C | Rapid rate of Periodontitis progression | CAL or RBL ≥2 mm over 5 years; tissue destruction exceeds expectations given the level of biofilm deposits |
Staging aims to classify the (I) severity of the patient’s disease based on the measurable amount of the destroyed tissue (clinical attachment loss/radiographic bone loss/number of teeth lost due to periodontitis), (II) complexity of local factors to assess management and (III) extent (localized, generalized or molar-incisor pattern). Grading intends to estimate the rate of periodontitis progression and report on risk factors (smoking quantity, diabetes control) to aid in predicting responsiveness to standard therapy, and potential impact on systemic health. The goal is to guide the intensity of therapy and monitoring of the patient. PD, periodontal pocket depth; CAL, clinical attachment loss; RBL, radiographic bone loss; VBL, vertical bone loss.
Peri-implant diseases: peri-implant mucositis and peri-implantitis
Teeth may be lost for different reasons, including but not limited to trauma, non-restorability, lack of supporting structure due to periodontitis, pulpal pathology and more. One replacement option is a surgically placed dental implant. With the increased use of dental implants for the replacement of missing teeth, there has been an increase in disease prevalence related to dental implants as well. Peri-implant mucositis is an inflammatory lesion of the soft tissues surrounding an endosseous implant without loss of supporting bone or continuing marginal bone loss (10). On the other hand, peri-implantitis is a pathological condition characterized by inflammation in the peri-implant connective tissue and progressive bone loss (11). Similar to periodontitis, peri-implantitis exhibits a chronic inflammatory response to the bacterial biofilm on the implant surface (12). Both peri-implant diseases are primarily caused by a disruption of the host-microbe homeostasis at the implant-mucosa interface.
Etiology of periodontal and peri-implant diseases
Periodontal disease is caused by the breakdown of periodontal host-microbe homeostasis, which can precipitate dysbiosis in susceptible hosts (13). Dysbiotic microbial communities in the plaque biofilm consist of keystone pathogens and pathobionts. Their synergistic virulence, in conjunction with the host response, leads to destructive inflammation, through escalating dysbiosis and inflammatory bone loss, leading to tooth loss and potential systemic complications. The plaque biofilm consists of mature colonies of spirochetes, filamentous organisms among others (14). Additionally, gram-negative bacteria are frequently isolated from the periodontal pockets (15). Periodontitis pathogens include Aggregatibacter actinomycetemcomitans, Eikenella corrodens, Fusobacterium nucleatum, Prevotella intermedia, Porphyromonas gingivalis, Tannerella forsythia and recently studied Desulfobulbus spp., Filifactor alocis and TM7 species; however, dental plaque consists of more than 800 known bacterial species which have been identified and this number is expected to rise with the advances in technology (16).
No one specific bacteria has been identified in peri-implant diseases; however, peri-implantitis was associated with higher counts of 19 bacterial species, including Porphyromonas gingivalis and Tannerella forsythia (11). A recent systematic review and meta-analysis assessing the microbiome in peri-implantitis lesions demonstrated a higher prevalence of Aggregatibacter actinomycetemcomitans and Prevotella intermedia in peri-implantitis compared to healthy implants (17). Knowledge of putative microorganisms may shed light on the pathogenesis and treatment of periodontal and peri-implant diseases. Diagnosis of these diseases is performed by clinical and radiographic examination; however, biomarkers in saliva or within the sulcular fluids of teeth and implants may offer quantitative and qualitative parameters to assist in diagnosis, prognosis and to compare and recommend treatment modalities (18).
Changes in the levels of the population of the species in the oral microbiome and within the plaque biofilm may initiate the host reaction which leads to periodontal disease. This soft plaque hardens by the precipitation of mineral salts, starting only days after plaque formation. Calculus is mineralized plaque and is a predisposing factor for periodontal inflammation because it is always covered in a biofilm of potential pathogens (19,20). The attachment of calculus on teeth or implants is mainly by mechanical locking onto surface irregularities. Therefore, removing the plaque biofilm and calculus deposits is considered a primary objective in periodontal treatment.
Periodontal and peri-implant disease management
The treatment goal in periodontal and peri-implant disease is to reduce the bacterial load, shift the bacterial composition of the biofilm, and improve cleanability of the affected teeth or implants. Reduction in the volume of the plaque biofilm is mainly accomplished by mechanical instrumentation. In most periodontitis patients, mechanical debridement and anti-infective chemotherapy can readily control the disease without the need for surgery. When managed, periodontitis patients may retain their dentition for a lifetime (21). The gold standard for treatment of periodontal disease is a system of mechanical debridement, home care and regular supportive maintenance periodontal therapy. Mechanical debridement alone for the control of periodontal disease may fail to remove pathogenic organisms because of accessibility and location and therefore may fail to control the disease (22). Similarly, if the patient has systemic or behavioral factors altering the host’s innate immune capacity for defense, then other solutions may be sought. Adjunct methods and materials may be utilized to aid the practitioner and the patient in their struggle against the putative microorganisms of periodontal disease.
Peri-implant mucositis is similar to gingivitis and is reversible once the plaque biofilm is managed (10). On the other hand, non-surgical therapy of peri-implantitis is often ineffective, and the treatment of choice is a surgical approach (23). Since mucositis is the precursor of peri-implantitis (24), it may be prudent to ensure the biofilm is controlled using adjunctive plaque control measures and antimicrobials. Although antimicrobial adjunct studies found low to moderate additive improvements in peri-implantitis therapy, the current information on the adjunctive use of locally or systemically administered antibiotics is insufficient to allow any firm specific recommendations for the use of these drugs (25,26).
Risk factors and indicators for disease progression
Factors which may affect host susceptibility to biofilm induced diseases may include smoking, poorly controlled diabetes, poor oral hygiene and lack of compliance with supportive therapy among others (10). Clinicians may suggest additive modalities to manage high risk patients. Adjunctive therapy with systemic antibiotics was found to improve the efficacy of non-surgical periodontal therapy, scaling and root planning (SRP), in the periodontal management of diabetic patients (27). Other clinical trials concluded that local antibiotic adjuncts offer added benefits even in well controlled diabetics (28).
Antimicrobials in periodontal and peri-implant therapy
Clinicians debate the utility of using antimicrobial adjuncts to mechanical treatment of periodontally diseased teeth or implants. Generally, the use of antimicrobials in periodontal or peri-implant therapy, and their types and dosages, has been empirical in nature. The lack of clear recommendations leads to controversy and each practitioner attempts to balance the cost-benefit as well as side-effects of these additive agents. Some periodontal organizations have worked to compile guidelines regarding the treatment of periodontitis.
The European Federation of Periodontology recently approved specific therapeutic guidelines for the treatment of periodontitis stages I to III which included antimicrobial and antiseptic indications (29). This review highlights current approaches to antimicrobial periodontal and peri-implant therapy and aims to recommend certain antimicrobials based on evidence in the literature. The adjunctive use of local statins, probiotics, systemic sub antimicrobial dose of doxycycline, systemic/local bisphosphonates, systemic/local non-steroid anti-inflammatory drugs, omega-3 polyunsaturated fatty acids and local metformin gel were not recommended. Consideration was recommended to antiseptic mouthwashes, and particularly chlorhexidine, as adjuncts to mechanical instrumentation in specific cases. Adjunctive antiseptics were advised to be considered for some patients during supportive periodontal therapy, in order to control inflammation of the gingiva. Locally delivered antibiotics were recommended to be considered as adjuncts to the subgingival SRP in some situations. However, when it comes to systemic antibiotics, their routine was not recommended, due to their negative effects, except in specific diagnoses.
Methods
A literature search was performed in the PubMed database, for articles published up to November 2020 using Medical Subject Heading search terms and free text terms and in different combinations. The search was conducted for each of the relevant topics addressed in this review.
The following terms and their variants were searched either individually or in combinations: periodontitis, gingivitis, peri-implant mucositis, peri-implantitis, antiseptics, antibiotics, antimicrobials, chlorhexidine, oral rinse, clinical practice guidelines, laser therapy, minocycline, therapeutic adjuncts. A manual search was performed to select recent articles for specific relevant topics. Data from the identified papers were analyzed and presented within the text or tables if indicated. The findings were presented in the form of a narrative review. Historically relevant publications were also included when deemed important. To be included in the review, studies had to be written in the English language, published in an international peer-reviewed journal, and be on humans. Furthermore, animal or in-vitro studies were supplemented by an additional search to find relevant supporting data. Citation tracking was completed using EndnoteTM, version 9 (Clarivate Analytics, Boston, MA, USA) for all identified studies included in the refined library. No restriction nor filters were placed on the type, availability nor year of publication for the included reports. A meta-analysis was not performed due to the heterogeneity of the included studies.
Adjuncts to non-surgical therapy in the management of periodontitis
Proposed adjuncts to mechanical treatment include local antimicrobials, systemic antimicrobials, lasers, oral rinses, sub-gingival and supra-gingival irrigations. Severe periodontal infections may be combatted using systemic antibiotics which inhibit or kill putative microorganisms. The microbial etiology of periodontal disease provides the rationale for the use of antimicrobials. Justification for adjunctive antibiotic use is to eliminate bacteria located in deep inaccessible pockets.
Systemic antibiotics
The position paper published by the American Academy of Periodontology in 2004 recommended indications for systemic antibiotic prescription for periodontal patients who do not respond to conventional therapy, patients with severe periodontal infections threatening oral and systemic health and medically compromised and susceptible patients (30). Antibiotics should only be prescribed after biofilm has been mechanically disrupted, not as the sole approach to treatment (31). Antibiotic administration changes the bacterial community in the periodontal sulcus thus modifying bacterial pathogenicity. Bacteria in subgingival biofilm are significantly more resistant to antibiotics if the biofilm is not mechanically disrupted (32). Nevertheless, it was demonstrated in a study that a combination of repeated systemic antibiotics may arrest the progression of chronic moderate-advanced progressive adult periodontitis as a sole therapy (33). Systemic antibiotics have the potential to produce adverse reactions that must be considered in balance with their expected benefits. There are warnings against the unrestricted use of antibiotics in treating periodontal diseases because of the emerging global public health issue of bacterial resistance (34).
Antibiotics with SRP offer greater pocket depth reduction and clinical attachment level gain especially in pockets greater than 6-mm deep and in severe forms of periodontitis (34). Adjunctive antibiotics are not usually prescribed for chronic mild-moderate periodontitis because the side effects outweigh the minor clinical benefits compared to SRP alone (34). An exception to the rule; however, is if the patient has recurrent, refractory or rapidly progressing periodontitis, is immunodeficient or is an uncontrolled diabetic (27,30,35). A study on type 1 diabetics having moderate to severe periodontitis concluded that the use of systemic doxycycline as an adjunct, provided more significant results than mechanical therapy alone (36). It is suggested that the cases with multiple deep pockets should first be treated by thorough SRP and adjunctive systemic antibiotics (37,38). Timing of systemic antibiotic administration (based on empirical knowledge) is to start the regimen one day before initial mechanical debridement, so the blood clot in the pocket will have antibiotic molecules at an effective concentration, then treat the contralateral side one to two days later (39).
A recent systematic review and meta-analysis concluded that the adjunctive use of systemic antibiotics in periodontal therapy resulted in significant benefits in clinical outcomes but with frequent adverse complications (40). Metronidazole alone or azithromycin alone yielded significant improvements in pocket depth reduction, clinical attachment level gain, bleeding on probing, pocket closure and frequency of residual pockets, however, the most favorable outcomes were found with the combination of amoxicillin with metronidazole (40). Since the putative microorganisms in the periodontal pocket respond differently to different classes of antibiotics, then one should consider the advantage of drug combinations (21,41). The combination of Amoxicillin (250 mg q8h) with Metronidazole (250 mg q8h) for 8 days is a common practice for young and middle-aged patients with severe forms of periodontitis (42). On the other hand, older patients as well as patients with penicillin allergies are prescribed Ciprofloxacin (500 mg q12h) with Metronidazole (500 mg q12h) for 8 days (42). These combinations of systemic antibiotics are effective against the major periodontopathic bacteria (42). Alternative prescription protocols for the Amoxicillin + Metronidazole combination were presented in the literature (43-48). Amoxicillin prescription ranged from 250 to 500 mg q8h and Metronidazole ranged from 250 to 500 mg q8h, while durations ranged from 7 to 14 days.
Acute periodontal lesions such as a periodontal abscess may spread causing systemic manifestations. If immediate or adequate drainage is not achieved or a systemic involvement is evident, therapy with systemic antimicrobials may be advised for 3 days (7,30,49,50). Another acute disease is necrotizing periodontitis which is an infectious condition occurring in individuals with a compromised host immune response (7). Mechanical debridement must be initiated immediately, and adjunctive oral rinses are indicated. If unsatisfactory response is evident or systemic effects are manifested then the use of systemic antibiotics may be considered (49). Another disease which may present in acute form is the endo-periodontal lesion which is a pathological communication between the endodontic and periodontal tissues of a tooth. Both root canal and periodontal tissues would require treatment, yet histologically, all periodontal abscess lesions are similar (7,49). Following mechanical instrumentation of both root canal and periodontal tissues, the need for systemic antibiotics must be assessed in a similar manner as the acute periodontal abscess; based on the presence of systemic manifestations (51).
Local delivery agents
Clinicians may prefer the use of antimicrobials locally delivered into persistent or recurrent localized deep periodontal pockets for an average additional 0.4 mm in pocket depth reduction and 0.3 mm in clinical attachment level gain (34). These antimicrobials may be in the form of a biodegradable sustained release solid inserted and left in the pocket or in the form of a liquid irrigation. Some clinicians prefer the use of local antimicrobials in the following situations: (I) ≥5 mm deep pockets, (II) where esthetics is a concern, especially the maxillary anterior region, rather than performing periodontal pocket reduction surgery, (III) where periodontal surgery did not achieve full disease resolution, refractory or recurrent periodontitis and (IV) medically compromised patients who would not be candidates for periodontal surgery.
Local antibiotics
Adverse side effects of systemic antibiotics may be avoided by using locally delivered antibiotics. The ability to deliver antibiotics locally into a diseased periodontal pocket offers direct benefit in the management of challenging cases. The effective concentration of local antibiotics was shown to be at least 100 times greater in the pocket than the systemic delivery of antibiotics (52,53). Local antibiotics along with SRP may be beneficial in recurrent or deeper periodontal pockets (54). The main local antibiotics studied are doxycycline and minocycline. Doxycycline showed a minimal additional benefit in some patients (55), while other studies did not find an additional benefit (56,57). Meanwhile, adjunctive use of minocycline delivered into the diseased deep pockets was shown to improve therapeutic outcomes when compared to SRP alone (53,58-60). Minocycline local delivery into a periodontal pocket is shown in Figure 1.

Local delivery of antiseptics
The adjunctive use of local antimicrobials such as subgingival biodegradable chlorhexidine chips was shown to offer improved pocket reduction compared to SRP alone in deep pockets (61,62). These chlorhexidine chips, (Periochip®), are inserted into the pocket and left in place to degrade with time. On the other hand, subgingival irrigation (lavage) of the pockets during SRP and supportive therapy appointments may be beneficial (21,63). One antimicrobial irrigation agent is povidone-iodine. This agent has been studied as an adjunct to SRP because of povidone-iodine’s broad-spectrum antimicrobial activity, low potential for developing resistance or adverse reactions, wide availability, ease of use and low cost (21,64). The addition of subgingival 10% povidone-iodine irrigation to conventional mechanical therapy was found to reduce total counts of periodontal pathogens with statistically significant reduction in deep pockets compared to SRP alone (65). The povidone-iodine antimicrobial may be used upon completion of SRP for a contact time of 5 minutes or used in dilution with the ultrasonic scaler coolant (21). Another topical antimicrobial is diluted sodium hypochlorite, the common household bleach. This agent possesses excellent antibacterial, antifungal and antiviral properties and has been used in dentistry for decades. A suggested concentration of sodium hypochlorite for periodontal pocket irrigation is ≤0.5% and was found to have no contraindications in the diluted form (66).
The use of subgingival antimicrobial irrigation is not routinely recommended according to the 2005 American Academy of Periodontology’s position paper due to insufficient evidence of an additive effect when used with mechanical therapy (67). However, it was noted that improved therapeutic results have been documented which show promise. Other subgingival irrigants utilized during non-surgical therapy include tetracycline, hydrogen peroxide and tetrapotassium peroxydiphosphate. A systematic review from 2005 evaluating the impact of local adjuncts to SRP in periodontal disease therapy concluded that hydrogen peroxide irrigant was the most promising antimicrobial in terms of pocket depth reduction (68) and that tetracycline and minocycline had the greatest positive results among locally administered antibiotics.
Supragingival irrigation on the other hand includes the use of a device that offers a pulsating stream of water. This oral irrigator may assist patients with inadequate manual cleaning skills or dexterity. Medicaments such as essential oils or chlorhexidine may be added to this water jet for the added benefit of introducing antimicrobials into hard-to-reach sites (69). The teeth staining effect of chlorhexidine is diminished due to this dilution with water. The benefit of using antimicrobials in the water jet device has been confirmed in gingivitis but remains unclear as an adjunct in treating periodontitis (67).
Adjuncts to surgical therapy in the management of periodontitis
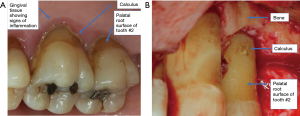
The surgical treatment of periodontitis aims to provide greater accessibility to mechanical debridement of root surfaces, elimination of fibrous periodontal tissue to improve pocket reduction, provide a harmonious osseous architecture for the gingiva to follow, allow greater accessibility of oral hygiene by the patient and maintenance performed by the dental practitioner. Calculus presence and gaining surgical access for subgingival calculus removal is shown in Figure 2. The use of a systemic antimicrobial during periodontal surgery has been evaluated for preventing post-operative infection by targeting of specific pathogenic bacterial profiles seen in refractory or aggressive periodontitis (70). Since the use of a systemic antimicrobial adjunct is to aid in disease resolution, its efficacy is measured by additional improvement in attachment level gain and reduction of pocket depths. Clinical trials have evaluated the potential benefit of using a systemic antibiotic in conjunction with periodontal surgery, but no significant differences were seen when compared to a placebo (71).
Many clinicians prescribe systemic antibiotics to reduce the risk of regenerative therapy failure due to bacterial infection. The use of an antibiotic for regenerative periodontal procedures is done so empirically, because of undesired effects in case of a membrane exposure during guided tissue/bone regeneration. However, a large-scale trial reported a generally low rate of post-operative infections (2.09%) after 1,053 periodontal surgical procedures whether or not peri-operative antibiotics were used (72). This retrospective study observed that the use of a regenerative membrane did not significantly increase infection rates compared to non-use of a membrane, 3.00% vs. 1.88%, respectively. Additionally, in an earlier retrospective study, the average rate of post-operative infections after periodontal surgeries was 1–2% with or without prophylactic antibiotic use (73). When specifically testing for the improvements of clinical parameters in regeneration with or without the use of amoxicillin, the greatest improvements were attributed to enamel matrix protein, not to the use of antibiotics (74). Enamel matrix protein is delivered in a sterile aqueous carrier of propylene glycol alginate (PGA) which may have beneficial antimicrobial effects by disturbing bacterial cell metabolism due to PGA’s low pH (75). Although there is conflicting evidence of any potential benefit for the use of an antimicrobial in conjunction with periodontal regeneration, the empirical use of an antimicrobial in previous clinical trials, supports their use with the aim of controlling the periodontal microflora and reducing the risk of a membrane exposure with subsequent infection, during the early post-surgical healing phase. As a result, until more clinical trials evaluating the use of an antimicrobial during periodontal surgery have been performed, no recommendation against the use of an antimicrobial can be made.
Adjuncts to therapy in the management of peri-implant diseases and conditions
In treating peri-implant mucositis, the efficacy of non-surgical therapy and at-home irrigation of these inflamed sites was reported to be advantageous (76,77). Chlorhexidine at 0.06% concentration using a powered subgingival irrigator, as well as an essential-oil mouth rinse may be beneficial for at-home antimicrobial agent use. Alternatively, locally delivered chlorhexidine chips had positive clinical results as well (76,78).
Controlling peri-implantitis includes elimination of the biofilm from the implant surfaces. However, the prosthesis and the implant’s rough and irregular surface may complicate efficient mechanical debridement. Non-surgical therapy initially performed is usually inadequate for treating peri-implantitis; therefore, surgery is indicated (23,77). Local antimicrobials may complement the initial non-surgical therapy. Reduction in pocket depths and bleeding on probing were reported with the adjunctive use of locally delivered minocycline microspheres or doxycycline, especially with repeated applications (76,77,79). Other locally delivered antimicrobials used in conjunction with mechanical decontamination include chlorhexidine gel or irrigation and hydrogen peroxide application (25,26,76,80-82). In fact, a recent multi-centered, randomized, clinical trial concluded that repeated bi-weekly delivery of chlorhexidine chips and supragingival plaque removal for 24 weeks significantly improved pocket depths and relative attachment gains in subjects with peri-implantitis (83).
Surgical techniques may include open flap debridement with mechanical and chemical decontamination of the exposed implant surface. Regenerative procedures to fill the bony defects caused by peri-implantitis may be successful as well (84,85). Peri-operative systemic antimicrobials, such as amoxicillin, metronidazole or amoxicillin plus clavulanic acid (Augmentin®), were prescribed in the majority of studies treating peri-implantitis yet; there is a lack of controlled studies evaluating their efficacy (77,85). One recent randomized controlled clinical trial concluded that systemic amoxicillin as an adjunct to mechanical debridement had a significant positive impact on the treatment of modified implant surfaces; yet did not affect the long-term outcome (86,87). Systemic antibiotics must be used with caution and their benefit balanced against their side effects. Intraoperative surface disinfection was reported to include citric acid, chlorhexidine, tetracycline hydrochloride and ethylenediamine tetra-acetate (EDTA) (76,77,85). No one chemical decontaminant was found to be superior; however, 3% hydrogen peroxide, applied on the implant surface for 2 minutes, was reported to be the most widely used (77,88).
Antimicrobials are commonly used during the initial procedure of implant placement. A 2-minute pre-operative rinse with 0.1% chlorhexidine can reduce the bacterial load by approximately 10-fold compared to sterile water (89). This is important for intra-operative autogenous bone collection and grafting during implant placement to ensure there are as few pathogens as possible in those grafted sites. The use of systemic antibiotics is recommended for immediate implant placement in an infected site (90). However, even under ordinary circumstances of implant placement, evidence suggests that prophylactic use of antibiotics reduces early failures (91-94). Different protocols have included pre-operative and/or post-operative use of systemic antibiotics. Reports indicated that for surgical implant placement, many practitioners used amoxicillin (1, 2 or 3 grams) (0–1 hour) pre-operatively only, while other clinicians added a 7-day post-operative course (94). A recent meta-analysis of surveys reported that other practitioners prescribe amoxicillin with clavulanic acid, penicillin V, azithromycin, clindamycin or metronidazole (95). The current evidence-based Cochrane review published in 2013 recommended a prophylactic regimen of amoxicillin 2 grams orally 1 hour prior to implant placement (93). It is noteworthy this review specified that giving antibiotics to 25 patients receiving implants will prevent one person from experiencing early implant loss.
On the other hand, augmentation procedures are sometimes performed in conjunction with, or prior to, implant placement. Procedures such as guided bone regeneration (GBR) and maxillary sinus elevation are usually accompanied by a regimen of antibiotics. Systemic antibiotic prophylaxis was found to be generally given in GBR (96). The probability of infection for most periodontal surgeries was found to be less than 6% with or without the use of antibiotics; yet, practitioners are likely to prescribe antibiotics with bone grafting procedures (97). A consensus report regarding direct sinus elevation surgery recommended prophylactic amoxicillin with clavulanic acid pre- and post-operatively to reduce the chance of graft infection (98). Patients allergic to penicillin would be prescribed clarithromycin with metronidazole. The antibiotics are to be started 24 hours before surgery and continued for 7 days. The report recommended another course of amoxicillin plus clavulanic acid or levofloxacin in the case of post-operative complications. Sinus infection management has been somewhat described in the literature (99,100). A recent review recommended doxycycline in case of penicillin allergy, at a dose of 100 mg twice daily for 7 days, starting 24 hours before the direct sinus elevation procedure (101).
At-home use of antimicrobials
In addition to the previously discussed oral irrigation jet devices along with the addition of medicaments, there are other approaches patients may follow to supplement their brushing and flossing. Antimicrobials may be used as oral rinses (mouthwashes) or dentifrice (toothpaste), anti-plaque agents. Other compounds may reduce the rate of calculus development; that are anti-calculus agents. These anti-calculus agents prevent the recurrence of periodontal disease after therapy because they demonstrated more effectiveness at preventing initial formation of biofilms (102). Mouthwashes have little penetration into the subgingival environment. Indications of mouth rinses include fresh breath, prevention of oral problems such as caries, gingivitis and tooth sensitivity (103). Potential side-effects of these at-home antimicrobials include staining, discomfort, numbness, oral desquamation, teeth erosion and altered taste.
A recent report described the daily at-home use of a teeth whitening foam/gel containing 0.1% cetylpyridinium chloride, 1.4% hydrogen peroxide, sodium bicarbonate, and antioxidant compounds on gingivitis (104). Subjects with gingivitis and mild-to-moderate periodontitis brushing daily with this foaming gel had a significant reduction in gingivitis compared to control subjects brushing with over-the-counter tooth paste. Brushing daily with this novel post foaming gel also resulted in greater reductions in periodontopathogens and inflammatory cytokines within the gingival crevicular fluid. Finally, clinicians should balance the advantages and disadvantages of recommending specific home-use products especially since these products are not meant to replace professional periodontal therapy.
Antiplaque agents
Chlorhexidine is an anti-plaque agent which has the advantages of substantivity and safety and has been extensively studied (105-109). Substantivity is the ability of chlorhexidine to adhere to teeth and oral mucosa extending its anti-plaque effects (66). Chlorhexidine can disturb bacterial cell membranes and is bactericidal in high concentrations (102). It is a broad-spectrum bactericidal antimicrobial which acts against Gram-positive and Gram-negative bacteria as well as yeast organisms. Chlorhexidine mouthwash as an adjunct to scaling and root planing can lead to slightly increased pocket depth reduction when compared to mechanical therapy alone, according to a recent meta-analysis (110). Additionally, the use of chlorhexidine mouth wash along with plaque control methods demonstrated a significant improvement in plaque and bleeding scores (111). At 0.2%, chlorhexidine oral rinse twice a day prevents plaque and gingivitis for 21 days without brushing, however, it is typically used in a 0.12% concentration which is also as clinically effective as 0.2%. Its use may be short term, intermittent or long term.
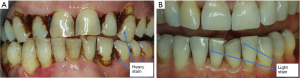
Teeth staining is one disadvantage which restricts the long-term use of chlorhexidine. Other side effects include tongue and mucosal surface staining, alterations of taste, desquamation of the mucosa, enlargement of the parotid and also increased calculus deposition supragingivally (112). Using 0.12% chlorhexidine gluconate for extended periods may lead to brown teeth staining which may be removed by professional polishing. However, long-term use may be recommended in debilitated patients or individuals unable to practice adequate oral hygiene measures. Brown teeth staining after the use of chlorhexidine mouthwash is shown in Figure 3. Chlorhexidine mouth rinse is typically used for several weeks after periodontal surgery. It is noteworthy that chlorhexidine contact with surgical sites for short periods of time prior to wound closure can have toxic effects on gingival fibroblasts and may negatively affect wound healing (113). Therefore, chlorhexidine is often initiated 24 hours after surgery.
Subgingival lavage using sodium hypochlorite has been previously discussed. Additionally, twice weekly oral rinsing with 0.25% sodium hypochlorite produced a significant reduction in bleeding on probing, even in deep unscaled pockets (114). Essential oils are used as oral rinses due to their antiseptic effect and their effectiveness in reducing plaque and gingivitis (115-117). One essential oil oral rinse brand found on the market (Listerine®) includes as ingredients: eucalyptol, menthol, methyl salicylate and thymol. Other mouth rinses contain ingredients which have shown effectiveness against plaque microorganisms and include amine fluoride, stannous fluoride, histatin, triclosan and mastic delivered in chewing gum form (118-121). Triclosan is a well-documented antibacterial used in toothpaste. However, due to recent safety concerns, triclosan was banned from all human hygiene biocidal products by the European Union and also banned from soap products by the United States Food and Drug Administration (122). Toothpaste containing triclosan is no longer commercially available according to the American Dental Association.
Anti-calculus agents
As calculus harbors pathogens in close proximity to gingival tissues, it is prudent to minimize the rate of calculus formation. Dentifrices containing calcium phosphate mineralization inhibitors (anti-calculus agents) have been shown to be highly effective in reducing the formation of dental calculus (123). Mucinase causes enzymatic dissolution of the organic matter in calculus, while pyrophosphate inhibits calculus crystal growth (124). Additionally, the clinical application of an amino acid buffered hypochlorite solution, Perisolv®, has recently shown promising in-vitro studies for the surface treatment of both periodontally involved teeth and diseased implants (125,126). This gel contains 0.95% sodium hypochlorite with amino acids such as glutamic acid, leucine, lysine. The subgingival application of this gel intends to disrupt bacterial biofilms and dissolve degenerated tissues with minimal negative effects on healthy tissues. Polypyrophosphate anion in dentifrice also demonstrated a positive effect in controlling calculus formation (127).
Laser therapy in periodontal and peri-implant diseases
Adjunctive laser therapy in periodontitis
Laser therapy was evaluated by an expert panel convened by the American Dental Association in 2015 (128,129). These experts agreed that in moderate to severe chronic periodontitis, the adjunctive use of photodynamic therapy (PDT) using diode lasers may offer a moderate additive benefit to SRP. This additional benefit was an average of 0.53 mm of further clinical attachment level gain. Antimicrobial PDT is light of an appropriate wavelength used in the presence of a specific photosensitizer to selectively eradicate target bacterial cells (130). Carbon dioxide laser has not shown statistical significance as to its additive effect with SRP and data were sparse and poor in quality (131).
Other laser types may be used in lieu of SRP. However, there was insufficient evidence that laser therapy was superior to SRP. Nd:YAG and Er:YAG lasers for treatment of chronic periodontitis may be equivalent to SRP with respect to reduction in probing depth and subgingival bacterial populations but not in attachment gain (132). Conversely, a recent review in laser therapy found the majority of the studies to be underpowered and exhibited significant heterogeneity in design; therefore, concrete conclusions could not be made (131). Finally, a recent guideline report by the European Federation of Periodontists did not recommend the use of lasers as an adjunct in conjunction to mechanical therapy as laser therapy did not prove to be a greater benefit compared to non-surgical therapy alone (29).
Adjunctive laser therapy in peri-implant mucositis and peri-implantitis
Treatment of peri-implant mucositis usually consists of mechanical debridement with or without antimicrobials. In surgical therapy of peri-implantitis, studies typically find that conventional mechanical instrumentation yields similar results as erbium and carbon dioxide lasers (76). However, non-surgical therapy of peri-implantitis such as laser treatment, may be initially attempted prior to surgery in reducing gingival inflammation and to evaluate the healing response (23,77). Er:YAG laser therapy offers a bactericidal effect (77). A systematic review concluded that Er:YAG laser treatment resulted in greater reduction in bleeding on probing compared to mechanical debridement with adjunctive irrigation using chlorhexidine (133). There is currently limited evidence that PDT with diode lasers may represent a possible alternative to adjunctive local antibiotics in patients with incipient peri-implantitis (134). A recent report found a similar benefit to PDT when compared with local minocycline application in the non-surgical treatment phase of peri-implantitis (135,136). Further research addressing laser therapy in periodontitis and peri-implantitis treatment should be pursued in future clinical trials.
Antimicrobial adjunct selection by condition
Clinicians seek clear recommendations for antimicrobial adjuncts in the treatment of each condition. Unfortunately, adjunctive antimicrobial therapeutic use remains a controversial issue due to the scarcity of large well-designed clinical trials on this topic. Guidelines for the use of antimicrobial adjuncts to non-surgical and surgical management of periodontal and peri-implant diseases and conditions are shown in Table 2. Some of these suggestions are evidence-based while others are empirical proposals but documented in the literature.
Table 2
| Condition | Antimicrobiala | Evidence level (low, moderate, high) | References |
|---|---|---|---|
| Gingivitis | Essential oils, CHX | Moderate | (69,102,105-107,116,117) |
| Periodontitis stage 1 & 2, grade A & B. (initial to moderate periodontitis) (for increased risk patientsb) | Essential oils, CHX, PI, NaOCl | Moderate | (63,102,108,110,116,117) |
| Periodontitis stage 3–4, grade B (severe periodontitis) (for increased risk patientsb) | Amox-Met, Cipro-Met, Doxy, SDD, AZ | High | (30,31,33,34,36,37,40,42,46-48,108,128,129) |
| CHX, PI, NaOCl. Locally delivered: minocycline, CHX-chip, DHG, PDT | High | (29,42,128,129) | |
| Periodontitis stage 3–4, grade C (advanced, rapidly progressing periodontitis) | Amox-Met, Cipro-Met, CHX, NaOCl, PI | High | (30,34,39,40,42-45) |
| Necrotizing periodontitis | MET, Amox-Met, AMXC, CHX, H2O2 | Moderate | (49) |
| Periodontal abscess & endo-periodontal lesions | Amox, AZ, AMXC, MET (in specific situations, such as in case of systemic manifestations, and for a 3-day duration) | High | (7,30,49-51) |
| Periodontal surgery (pre-operatively) | CHX for 1 min | Moderate | (89) |
| Periodontal non-regenerative surgery (post-operatively) | CHX for 1 min TID for 2–4 weeks after surgery | Moderate | (109) |
| Regenerative procedures (prophylaxis) (GBR, GTR) | Amox, CHX | Low | (89,96,109) |
| Sinus elevation procedures (prophylaxis) | AMXC 875/125 g PO q12h for 7 days starting 24 h before surgery | Low | (98,101) |
| Or clarithromycin 250 mg PO BID + Metro 500 mg PO TID for 7 days starting 24 h before surgery | |||
| Or Doxy 100 mg BID for 7 days starting 24 h before surgery | |||
| Sinus elevation post-operative infection | AMXC 1 g PO TID + metronidazole 500 mg TID for 7–10 days | Moderate | (98-101) |
| Or Doxy 100 mg BID for 14 days | |||
| Or levofloxacin 500 mg PO daily for 5–10 days | |||
| Implant placement (pre-operatively) | CHX for 1 min before surgery. Amox 2 g PO 1 h before surgery, or 600 mg clindamycin 1 h before surgery | Low | (89,93,95) |
| Implant placement (post-operatively) | CHX for 1 min TID for 2 weeks after surgery | Low | – |
| Peri-implant mucositis | Locally delivered: CHX chip. At-home: essential-oils rinse, 0.06% CHX using a powered subgingival irrigator | Low | (76-78) |
| Peri-implantitis (non-surgical therapy) | Locally delivered: minocycline, doxycycline, CHX gel, CHX chip, H2O2, PDT | Low | (25,26,76,77) |
| Peri-implantitis (resective or regenerative surgical therapy) | Locally delivered: CHX gel, H2O2, citric acid, EDTA | Moderate | (76,77,85) |
| Peri-implantitis (regenerative surgical therapy) | Systemic antibiotics: Amox | Low | (26,82) |
Antimicrobials proposed in this table are to be used on a case-by-case basis and clinicians must weigh their benefits against their risks. The treatment goal in periodontal and peri-implant diseases and conditions is to reduce the bacterial load and improve cleanability of the affected sites. The systemic antimicrobials are listed in order of highest to lowest recommendation. If patients are allergic to a specific antibiotic, then the following option listed may be used. Combinations of systemic and local antimicrobials may be used at the clinician’s discretion. Evidence level definitions; Low: there is a low level of certainty of benefits and agreement in published literature, Moderate: there is a moderate level of certainty of benefits and agreement in published literature, High: there is a high level of certainty of benefits and agreement in published literature. a, abbreviations and dosages: essential oils: thymol, eucalyptol, menthol, and methyl salicylate (example: Listerine®). CHX: 0.12% chlorhexidine gluconate mouthwash; rinse for 1 minute BID for 2 weeks. PI: 10% povidone-iodine; subgingival irrigation for 5 minutes. NaOCl: freshly diluted (0.1–0.25%) sodium hypochlorite mouthwash for 30 seconds; twice weekly. Amox-Met: systemic amoxicillin 500 mg q8h and metronidazole 250 mg q8h for 7 days. (alternative dosages may be recommended). Cipro-Met: systemic ciprofloxacin and metronidazole at 500 mg each q8h for 8 days. (alternative dosages may be recommended). Doxy: doxycycline (100 mg/day for 15 days). SDD: systemic sub-antimicrobial dose doxycycline (20 mg BID for 3–9 months). AZ: Azithromycin 500 mg qd for 3 days. Minocycline: minocycline microspheres (Arestin®). CHX-chip: chlorhexidine chip. DHG: doxycycline hyclate gel (example: Atridox®). PDT: photodynamic therapy with diode laser. AMXC: amoxicillin plus clavulanic acid. Example: Augmentin® 500/125 mg q8h for 8 days. H2O2: 1.5% hydrogen peroxide mouthwash. Amox: amoxicillin 500 mg q8h for 7 days. MET: metronidazole 250 mg q8h. GBR, guided bone regeneration; GTR, guided tissue regeneration; EDTA, ethylenediamine tetra‐acetate. b, increased risk patients include those individuals who have rapidly progressing attachment loss, invasive subgingival pathogens, multiple deep pockets, recurrent deep pockets, refractory disease, are immunocompromised, uncontrolled diabetics or heavy smokers.
Conclusions
Periodontal and peri-implant diseases are mainly managed by manual instrumentation to reduce the bacterial load and improve at-home cleanability by the patient. Therapeutic adjuncts may be considered in patients with risk factors such as uncontrolled diabetes, heavy smokers, rapidly progressing attachment loss, multiple deep pockets and immunocompromised individuals. Adjuncts to mechanical therapy include antimicrobials which assist in reducing the bacterial insult and spread. Locally applied antimicrobials include at-home oral rinses and irrigations, or professionally administered intrasulcular antimicrobials or subgingival irrigants. Other adjuncts to mechanical debridement of periodontally diseased teeth or implants include lasers such as PDT. Specific surgical procedures may also benefit from antimicrobial use to prevent post-surgical infection. Evidence based recommendations are present for some situations; however, the literature is sparse in regenerative procedure recommendations. Future trials should address the value of systemic or local antimicrobial use with periodontal regenerative procedures.
Acknowledgments
The authors are grateful to Dr. Miguel Sanchez, Division of Periodontology, Department of Developmental and Surgical Sciences at the University of Minnesota School of Dentistry, for his assistance.
Funding: This research received no external funding but did receive a research support grant from the University of Minnesota, Division of Periodontology, L. Wolff.
Footnote
Peer Review File: Available at https://fomm.amegroups.org/article/view/10.21037/fomm-20-84/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.org/article/view/10.21037/fomm-20-84/coif). JAP is a military Service member (or employee of the U.S. Government). This work was prepared as part of JAP’s official duties. Title 17, U.S.C., §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C., §101 defines a U.S. Government work as a work prepared by a military Service member or employee of the U.S. Government as part of that person’s official duties. The other authors have no conflicts of interest to declare.
Disclaimer: The views expressed in this review are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chapple ILC, Mealey BL, Van Dyke TE, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89:S74-84. [Crossref] [PubMed]
- Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 2018;45:S162-70. [Crossref] [PubMed]
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999;4:1-6. [Crossref] [PubMed]
- Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Periodontol 2018;89:S1-8. [Crossref] [PubMed]
- Jepsen S, Caton JG, Albandar JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89:S237-48. [Crossref] [PubMed]
- Albandar JM, Susin C, Hughes FJ. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J Periodontol 2018;89:S183-203. [Crossref] [PubMed]
- Herrera D, Retamal-Valdes B, Alonso B, et al. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J Periodontol 2018;89:S85-S102. [Crossref] [PubMed]
- Tonetti MS, Sanz M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J Clin Periodontol 2019;46:398-405. [Crossref] [PubMed]
- Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol 2018;89:S159-72. [Crossref] [PubMed]
- Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Periodontol 2018;89:S257-66. [Crossref] [PubMed]
- Schwarz F, Derks J, Monje A, et al. Peri-implantitis. J Periodontol 2018;89:S267-90. [Crossref] [PubMed]
- Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000 2010;53:167-81. [Crossref] [PubMed]
- Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 2014;35:3-11. [Crossref] [PubMed]
- Bernimoulin JP. Recent concepts in plaque formation. J Clin Periodontol 2003;30:7-9. [Crossref] [PubMed]
- Patini R, Staderini E, Lajolo C, et al. Relationship between oral microbiota and periodontal disease: a systematic review. Eur Rev Med Pharmacol Sci 2018;22:5775-88. [PubMed]
- Filoche S, Wong L, Sissons CH. Oral biofilms: emerging concepts in microbial ecology. J Dent Res 2010;89:8-18. [Crossref] [PubMed]
- Sahrmann P, Gilli F, Wiedemeier DB, et al. The Microbiome of Peri-Implantitis: A Systematic Review and Meta-Analysis. Microorganisms 2020;8:661. [Crossref] [PubMed]
- Alassy H, Parachuru P, Wolff L. Peri-Implantitis Diagnosis and Prognosis Using Biomarkers in Peri-Implant Crevicular Fluid: A Narrative Review. Diagnostics (Basel) 2019;9:214. [Crossref] [PubMed]
- Friskopp J, Hammarstrom L. A comparative, scanning electron microscopic study of supragingival and subgingival calculus. J Periodontol 1980;51:553-62. [Crossref] [PubMed]
- Listgarten MA, Ellegaard B. Electron microscopic evidence of a cellular attachment between junctional epithelium and dental calculus. J Periodontal Res 1973;8:143-50. [Crossref] [PubMed]
- Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodontal Res 2002;37:389-98. [Crossref] [PubMed]
- Santos RS, Macedo RF, Souza EA, et al. The use of systemic antibiotics in the treatment of refractory periodontitis: A systematic review. J Am Dent Assoc 2016;147:577-85. [Crossref] [PubMed]
- Englezos E, Cosyn J, Koole S, et al. Resective Treatment of Peri-implantitis: Clinical and Radiographic Outcomes After 2 Years. Int J Periodontics Restorative Dent 2018;38:729-35. [Crossref] [PubMed]
- Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89:S313-8. [Crossref] [PubMed]
- van Winkelhoff AJ. Antibiotics in the treatment of peri-implantitis. Eur J Oral Implantol 2012;5:S43-50. [PubMed]
- Javed F, Alghamdi AS, Ahmed A, et al. Clinical efficacy of antibiotics in the treatment of peri-implantitis. Int Dent J 2013;63:169-76. [Crossref] [PubMed]
- Grellmann AP, Sfreddo CS, Maier J, et al. Systemic antimicrobials adjuvant to periodontal therapy in diabetic subjects: a meta-analysis. J Clin Periodontol 2016;43:250-60. [Crossref] [PubMed]
- Rovai ES, Souto ML, Ganhito JA, et al. Efficacy of Local Antimicrobials in the Non-Surgical Treatment of Patients with Periodontitis and Diabetes: A Systematic Review. J Periodontol 2016;87:1406-17. [Crossref] [PubMed]
- Sanz M, Herrera D, Kebschull M, et al. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol 2020;47:4-60. [Crossref] [PubMed]
- Slots J, Research S, Therapy C. Systemic antibiotics in periodontics. J Periodontol 2004;75:1553-65. [Crossref] [PubMed]
- Walters J, Lai PC. Should Antibiotics Be Prescribed to Treat Chronic Periodontitis? Dent Clin North Am 2015;59:919-33. [Crossref] [PubMed]
- Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis 2015;34:877-86. [Crossref] [PubMed]
- López NJ, Gamonal JA, Martinez B. Repeated metronidazole and amoxicillin treatment of periodontitis. A follow-up study. J Periodontol 2000;71:79-89. [Crossref] [PubMed]
- Jepsen K, Jepsen S. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000 2016;71:82-112. [Crossref] [PubMed]
- Slots J, Rams TE. New views on periodontal microbiota in special patient categories. J Clin Periodontol 1991;18:411-20. [Crossref] [PubMed]
- Llambés F, Silvestre FJ, Hernandez-Mijares A, et al. Effect of non-surgical periodontal treatment with or without doxycycline on the periodontium of type 1 diabetic patients. J Clin Periodontol 2005;32:915-20. [Crossref] [PubMed]
- Cionca N, Giannopoulou C, Ugolotti G, et al. Amoxicillin and metronidazole as an adjunct to full-mouth scaling and root planing of chronic periodontitis. J Periodontol 2009;80:364-71. [Crossref] [PubMed]
- Mombelli A, Cionca N, Almaghlouth A. Does adjunctive antimicrobial therapy reduce the perceived need for periodontal surgery? Periodontol 2000 2011;55:205-16. [Crossref] [PubMed]
- Kaner D, Christan C, Dietrich T, et al. Timing affects the clinical outcome of adjunctive systemic antibiotic therapy for generalized aggressive periodontitis. J Periodontol 2007;78:1201-8. [Crossref] [PubMed]
- Teughels W, Feres M, Oud V, et al. Adjunctive effect of systemic antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J Clin Periodontol 2020;47:257-81. [Crossref] [PubMed]
- Tenenbaum H, Jehl F, Gallion C, et al. Amoxicillin and clavulanic acid concentrations in gingival crevicular fluid. J Clin Periodontol 1997;24:804-7. [Crossref] [PubMed]
- Slots J. Primer on etiology and treatment of progressive/severe periodontitis: A systemic health perspective. Periodontol 2000 2020;83:272-6. [Crossref] [PubMed]
- Guerrero A, Griffiths GS, Nibali L, et al. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: a randomized placebo-controlled clinical trial. J Clin Periodontol 2005;32:1096-107. [Crossref] [PubMed]
- Mestnik MJ, Feres M, Figueiredo LC, et al. Short-term benefits of the adjunctive use of metronidazole plus amoxicillin in the microbial profile and in the clinical parameters of subjects with generalized aggressive periodontitis. J Clin Periodontol 2010;37:353-65. [Crossref] [PubMed]
- Silva-Senem MX, Heller D, Varela VM, et al. Clinical and microbiological effects of systemic antimicrobials combined to an anti-infective mechanical debridement for the management of aggressive periodontitis: a 12-month randomized controlled trial. J Clin Periodontol 2013;40:242-51. [Crossref] [PubMed]
- Harks I, Koch R, Eickholz P, et al. Is progression of periodontitis relevantly influenced by systemic antibiotics? A clinical randomized trial. J Clin Periodontol 2015;42:832-42. [Crossref] [PubMed]
- Ehmke B, Moter A, Beikler T, et al. Adjunctive antimicrobial therapy of periodontitis: long-term effects on disease progression and oral colonization. J Periodontol 2005;76:749-59. [Crossref] [PubMed]
- Feres M, Soares GM, Mendes JA, et al. Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis: a 1-year double-blinded, placebo-controlled, randomized clinical trial. J Clin Periodontol 2012;39:1149-58. [Crossref] [PubMed]
- Herrera D, Alonso B, de Arriba L, et al. Acute periodontal lesions. Periodontol 2000 2014;65:149-77. [Crossref] [PubMed]
- Herrera D, Roldan S, O'Connor A, et al. The periodontal abscess (II). Short-term clinical and microbiological efficacy of 2 systemic antibiotic regimes. J Clin Periodontol 2000;27:395-404. [Crossref] [PubMed]
- Lockhart PB, Tampi MP, Abt E, et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J Am Dent Assoc 2019;150:906-921.e12. [Crossref] [PubMed]
- Freeman E, Ellen RP, Thompson G, et al. Gingival crevicular fluid concentration and side effects of minocycline: a comparison of two dose regimens. J Periodontol 1992;63:13-8. [Crossref] [PubMed]
- Williams RC, Paquette DW, Offenbacher S, et al. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J Periodontol 2001;72:1535-44. [Crossref] [PubMed]
- Etienne D. Locally delivered antimicrobials for the treatment of chronic periodontitis. Oral Dis 2003;9:45-50. [Crossref] [PubMed]
- Martorelli de Lima AF, Cury CC, Palioto DB, et al. Therapy with adjunctive doxycycline local delivery in patients with type 1 diabetes mellitus and periodontitis. J Clin Periodontol 2004;31:648-53. [Crossref] [PubMed]
- Tomasi C, Wennstrom JL. Locally delivered doxycycline as an adjunct to mechanical debridement at retreatment of periodontal pockets: outcome at furcation sites. J Periodontol 2011;82:210-8. [Crossref] [PubMed]
- Garrett S, Johnson L, Drisko CH, et al. Two multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis. J Periodontol 1999;70:490-503. [Crossref] [PubMed]
- Skaleric U, Schara R, Medvescek M, et al. Periodontal treatment by Arestin and its effects on glycemic control in type 1 diabetes patients. J Int Acad Periodontol 2004;6:160-5. [PubMed]
- Grossi SG, Goodson JM, Gunsolley JC, et al. Mechanical therapy with adjunctive minocycline microspheres reduces red-complex bacteria in smokers. J Periodontol 2007;78:1741-50. [Crossref] [PubMed]
- Goodson JM, Gunsolley JC, Grossi SG, et al. Minocycline HCl microspheres reduce red-complex bacteria in periodontal disease therapy. J Periodontol 2007;78:1568-79. [Crossref] [PubMed]
- Duarte PM, de Mendonca AC, Maximo MB, et al. Effect of anti-infective mechanical therapy on clinical parameters and cytokine levels in human peri-implant diseases. J Periodontol 2009;80:234-43. [Crossref] [PubMed]
- Jeffcoat MK, Bray KS, Ciancio SG, et al. Adjunctive use of a subgingival controlled-release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol 1998;69:989-97. [Crossref] [PubMed]
- Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000 2017;75:7-23. [Crossref] [PubMed]
- Hoang T, Jorgensen MG, Keim RG, et al. Povidone-iodine as a periodontal pocket disinfectant. J Periodontal Res 2003;38:311-7. [Crossref] [PubMed]
- Sahrmann P, Puhan MA, Attin T, et al. Systematic review on the effect of rinsing with povidone-iodine during nonsurgical periodontal therapy. J Periodontal Res 2010;45:153-64. [Crossref] [PubMed]
- Slots J. Low-cost periodontal therapy. Periodontol 2000 2012;60:110-37. [Crossref] [PubMed]
- Greenstein G, Research S. Therapy Committee of the American Academy of P. Position paper: The role of supra- and subgingival irrigation in the treatment of periodontal diseases. J Periodontol 2005;76:2015-27. [Crossref] [PubMed]
- Bonito AJ, Lux L, Lohr KN. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J Periodontol 2005;76:1227-36. [Crossref] [PubMed]
- Chaves ES, Kornman KS, Manwell MA, et al. Mechanism of irrigation effects on gingivitis. J Periodontol 1994;65:1016-21. [Crossref] [PubMed]
- Herrera D, Alonso B, Leon R, et al. Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. J Clin Periodontol 2008;35:45-66. [Crossref] [PubMed]
- Haffajee AD, Dzink JL, Socransky SS. Effect of modified Widman flap surgery and systemic tetracycline on the subgingival microbiota of periodontal lesions. J Clin Periodontol 1988;15:255-62. [Crossref] [PubMed]
- Powell CA, Mealey BL, Deas DE, et al. Post-surgical infections: prevalence associated with various periodontal surgical procedures. J Periodontol 2005;76:329-33. [Crossref] [PubMed]
- Pack PD, Haber J. The incidence of clinical infection after periodontal surgery. A retrospective study. J Periodontol 1983;54:441-3. [Crossref] [PubMed]
- Sculean A, Blaes A, Arweiler N, et al. The effect of postsurgical antibiotics on the healing of intrabony defects following treatment with enamel matrix proteins. J Periodontol 2001;72:190-5. [Crossref] [PubMed]
- Arweiler NB, Auschill TM, Donos N, et al. Antibacterial effect of an enamel matrix protein derivative on in vivo dental biofilm vitality. Clin Oral Investig 2002;6:205-9. [Crossref] [PubMed]
- Figuero E, Graziani F, Sanz I, et al. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000 2014;66:255-73. [Crossref] [PubMed]
- Renvert S, Polyzois I. Treatment of pathologic peri-implant pockets. Periodontol 2000 2018;76:180-90. [Crossref] [PubMed]
- Sahrmann P, Bettschart C, Wiedemeier DB, et al. Treatment of Peri-Implant Mucositis with Repeated Application of Chlorhexidine Chips or Gel during Supportive Therapy - A Randomized Clinical Trial. Dent J (Basel) 2019;7:115. [Crossref] [PubMed]
- Renvert S, Lessem J, Dahlen G, et al. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: a randomized clinical trial. J Periodontol 2008;79:836-44. [Crossref] [PubMed]
- Crespi R, Marconcini S, Crespi G, et al. Nonsurgical Treatment of Peri-implantitis Without Eliminating Granulation Tissue: A 3-Year Study. Implant Dent 2019;28:4-10. [Crossref] [PubMed]
- Leonhardt A, Dahlén G, Renvert S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J Periodontol 2003;74:1415-22. [Crossref] [PubMed]
- Smeets R, Henningsen A, Jung O, et al. Definition, etiology, prevention and treatment of peri-implantitis--a review. Head Face Med 2014;10:34. [Crossref] [PubMed]
- Machtei EE, Romanos G, Kang P, et al. Repeated delivery of chlorhexidine chips for the treatment of peri-implantitis: A multicenter, randomized, comparative clinical trial. J Periodontol 2021;92:11-20. [Crossref] [PubMed]
- Sahrmann P, Attin T, Schmidlin PR. Regenerative treatment of peri-implantitis using bone substitutes and membrane: a systematic review. Clin Implant Dent Relat Res 2011;13:46-57. [Crossref] [PubMed]
- Roccuzzo M, Layton DM, Roccuzzo A, et al. Clinical outcomes of peri-implantitis treatment and supportive care: A systematic review. Clin Oral Implants Res 2018;29:331-50. [Crossref] [PubMed]
- Carcuac O, Derks J, Charalampakis G, et al. Adjunctive Systemic and Local Antimicrobial Therapy in the Surgical Treatment of Peri-implantitis: A Randomized Controlled Clinical Trial. J Dent Res 2016;95:50-7. [Crossref] [PubMed]
- Carcuac O, Derks J, Abrahamsson I, et al. Surgical treatment of peri-implantitis: 3-year results from a randomized controlled clinical trial. J Clin Periodontol 2017;44:1294-303. [Crossref] [PubMed]
- Lagervall M, Jansson LE. Treatment outcome in patients with peri-implantitis in a periodontal clinic: a retrospective study. J Periodontol 2013;84:1365-73. [Crossref] [PubMed]
- Young MP, Korachi M, Carter DH, et al. The effects of an immediately pre-surgical chlorhexidine oral rinse on the bacterial contaminants of bone debris collected during dental implant surgery. Clin Oral Implants Res 2002;13:20-9. [Crossref] [PubMed]
- Waasdorp JA, Evian CI, Mandracchia M. Immediate placement of implants into infected sites: a systematic review of the literature. J Periodontol 2010;81:801-8. [Crossref] [PubMed]
- Keenan JR, Veitz-Keenan A. Antibiotic prophylaxis for dental implant placement? Evid Based Dent 2015;16:52-3. [Crossref] [PubMed]
- Chrcanovic BR, Albrektsson T, Wennerberg A. Prophylactic antibiotic regimen and dental implant failure: a meta-analysis. J Oral Rehabil 2014;41:941-56. [Crossref] [PubMed]
- Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications. Cochrane Database Syst Rev 2013;2013:CD004152. [Crossref] [PubMed]
- Canullo L, Troiano G, Sbricoli L, et al. The Use of Antibiotics in Implant Therapy: A Systematic Review and Meta-Analysis with Trial Sequential Analysis on Early Implant Failure. Int J Oral Maxillofac Implants 2020;35:485-94. [Crossref] [PubMed]
- Rodríguez Sánchez F, Arteagoitia I, Teughels W, et al. Antibiotic dosage prescribed in oral implant surgery: A meta-analysis of cross-sectional surveys. PLoS One 2020;15:e0236981. [Crossref] [PubMed]
- Esposito M, Grusovin MG, Coulthard P, et al. The efficacy of various bone augmentation procedures for dental implants: a Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants 2006;21:696-710. [PubMed]
- Hai JH, Lee C, Kapila YL, et al. Antibiotic prescribing practices in periodontal surgeries with and without bone grafting. J Periodontol 2020;91:508-15. [Crossref] [PubMed]
- Testori T, Drago L, Wallace SS, et al. Prevention and treatment of postoperative infections after sinus elevation surgery: clinical consensus and recommendations. Int J Dent 2012;2012:365809. [Crossref] [PubMed]
- Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012;54:e72-112. [Crossref] [PubMed]
- Johnson TM, Lincicum AR. Management of Wound Infection and Acute Bacterial Rhinosinusitis After Sinus Elevation Surgery: A Case Report. Clin Adv Periodontics 2018;8:54-60. [PubMed]
- Akers JA, Johnson TM, Hill RB, et al. Rational Prophylactic Antibiotic Selection for Sinus Elevation Surgery. Clin Adv Periodontics 2020;10:42-55. [Crossref] [PubMed]
- Baehni PC, Takeuchi Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis 2003;9:23-9. [Crossref] [PubMed]
- Moran JM. Home-use oral hygiene products: mouthrinses. Periodontol 2000 2008;48:42-53. [Crossref] [PubMed]
- Levine JB, Goncalves J, Nguyen D, et al. Efficacy of a novel post-foaming dental gel on gingival inflammation: A randomized controlled clinical trial. J Periodontol 2020;91:1569-83. [Crossref] [PubMed]
- Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res 1970;5:79-83. [Crossref] [PubMed]
- Bretz WA, Valente MI, Djahjah C, et al. Chlorhexidine varnishes prevent gingivitis in adolescents. ASDC J Dent Child 2000;67:399-402, 374.
- Caton JG, Blieden TM, Lowenguth RA, et al. Comparison between mechanical cleaning and an antimicrobial rinse for the treatment and prevention of interdental gingivitis. J Clin Periodontol 1993;20:172-8. [Crossref] [PubMed]
- Walker CB, Karpinia K, Baehni P. Chemotherapeutics: antibiotics and other antimicrobials. Periodontol 2000 2004;36:146-65. [Crossref] [PubMed]
- Sanz M, Newman MG, Anderson L, et al. Clinical enhancement of post-periodontal surgical therapy by a 0.12% chlorhexidine gluconate mouthrinse. J Periodontol 1989;60:570-6. [Crossref] [PubMed]
- da Costa LF, Amaral C, Barbirato DDS, et al. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: A meta-analysis. J Am Dent Assoc 2017;148:308-18. [Crossref] [PubMed]
- Van Strydonck DA, Slot DE, Van der Velden U, et al. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol 2012;39:1042-55. [Crossref] [PubMed]
- Addy M. Oral hygiene products: potential for harm to oral and systemic health? Periodontol 2000 2008;48:54-65. [Crossref] [PubMed]
- Mariotti AJ, Rumpf DA. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. J Periodontol 1999;70:1443-8. [Crossref] [PubMed]
- Gonzalez S, Cohen CL, Galvan M, et al. Gingival bleeding on probing: relationship to change in periodontal pocket depth and effect of sodium hypochlorite oral rinse. J Periodontal Res 2015;50:397-402. [Crossref] [PubMed]
- Santos A. Evidence-based control of plaque and gingivitis. J Clin Periodontol 2003;30:13-6. [Crossref] [PubMed]
- Jackson RJ. Metal salts, essential oils and phenols--old or new? Periodontol 2000 1997;15:63-73. [Crossref] [PubMed]
- Riep BG, Bernimoulin JP, Barnett ML. Comparative antiplaque effectiveness of an essential oil and an amine fluoride/stannous fluoride mouthrinse. J Clin Periodontol 1999;26:164-8. [Crossref] [PubMed]
- Horwitz J, Machtei EE, Peled M, et al. Amine fluoride/stannous fluoride and chlorhexidine mouthwashes as adjuncts to surgical periodontal therapy: a comparative study. J Periodontol 2000;71:1601-6. [Crossref] [PubMed]
- Van Dyke T, Paquette D, Grossi S, et al. Clinical and microbial evaluation of a histatin-containing mouthrinse in humans with experimental gingivitis: a phase-2 multi-center study. J Clin Periodontol 2002;29:168-76. [Crossref] [PubMed]
- Yates R, Shearer BH, Huntington E, et al. A method to compare four mouthrinses: time to gingivitis level as the primary outcome variable. J Clin Periodontol 2002;29:519-23. [Crossref] [PubMed]
- Takahashi K, Fukazawa M, Motohira H, et al. A pilot study on antiplaque effects of mastic chewing gum in the oral cavity. J Periodontol 2003;74:501-5. [Crossref] [PubMed]
- Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Rev 2017;20:447-69. [Crossref] [PubMed]
- Liu H, Segreto V, Baker R, et al. Anticalculus efficacy and safety of a novel whitening dentifrice containing sodium hexametaphosphate: a controlled six-month clinical trial. J Clin Dent 2002;13:25-8. [PubMed]
- Jin Y, Yip HK. Supragingival calculus: formation and control. Crit Rev Oral Biol Med 2002;13:426-41. [Crossref] [PubMed]
- Schmidlin PR, Fujioka-Kobayashi M, Mueller HD, et al. Effects of air polishing and an amino acid buffered hypochlorite solution to dentin surfaces and periodontal ligament cell survival, attachment, and spreading. Clin Oral Investig 2017;21:1589-98. [Crossref] [PubMed]
- Kubasiewicz-Ross P, Hadzik J, Gedrange T, et al. Antimicrobial Efficacy of Different Decontamination Methods as Tested on Dental Implants with Various Types of Surfaces. Med Sci Monit 2020;26:e920513. [Crossref] [PubMed]
- White DJ, Gerlach RW. Anticalculus effects of a novel, dual-phase polypyrophosphate dentifrice: chemical basis, mechanism, and clinical response. J Contemp Dent Pract 2000;1:1-19. [Crossref] [PubMed]
- Smiley CJ, Tracy SL, Abt E, et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc 2015;146:525-35. [Crossref] [PubMed]
- Smiley CJ, Tracy SL, Abt E, et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc 2015;146:508-24.e5. [Crossref] [PubMed]
- Betsy J, Prasanth CS, Baiju KV, et al. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: a randomized controlled clinical trial. J Clin Periodontol 2014;41:573-81. [Crossref] [PubMed]
- Cobb CM. Lasers and the treatment of periodontitis: the essence and the noise. Periodontol 2000 2017;75:205-95. [Crossref] [PubMed]
- Cobb CM. Lasers in periodontics: a review of the literature. J Periodontol 2006;77:545-64. [Crossref] [PubMed]
- Muthukuru M, Zainvi A, Esplugues EO, et al. Non-surgical therapy for the management of peri-implantitis: a systematic review. Clin Oral Implants Res 2012;23:77-83. [Crossref] [PubMed]
- Sculean A, Aoki A, Romanos G, et al. Is Photodynamic Therapy an Effective Treatment for Periodontal and Peri-Implant Infections? Dent Clin North Am 2015;59:831-58. [Crossref] [PubMed]
- Bassetti M, Schar D, Wicki B, et al. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res 2014;25:279-87. [Crossref] [PubMed]
- Schär D, Ramseier CA, Eick S, et al. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin Oral Implants Res 2013;24:104-10. [Crossref] [PubMed]
Cite this article as: Alassy H, Pizarek JA, Kormas I, Pedercini A, Wolff LF. Antimicrobial adjuncts in the management of periodontal and peri-implant diseases and conditions: a narrative review. Front Oral Maxillofac Med 2021;3:16.

