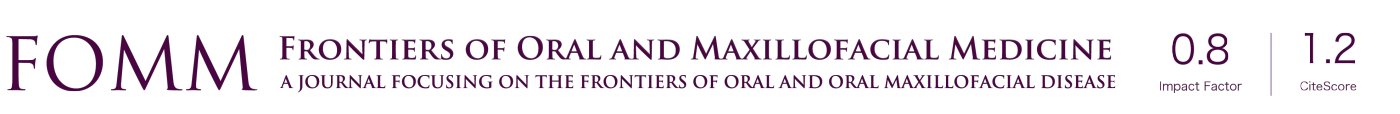Emerging technologies in the surgical management and treatment of the temporomandibular joint: a narrative review
Introduction
The temporomandibular joint (TMJ) is a bilateral synovial articulation between the temporal bone of the skull above and the mandible below and functions to allow the individual to chew, bite, and speak. Dysfunctions of the TMJ can have serious consequences in the day to day activities of an individual. Treatment of advanced temporomandibular joint disorders (TMJD) may necessitate surgical intervention to address functional concerns of the TMJ. Surgical treatment may include: intra-articular injection of therapeutic material, arthrocentesis, arthroscopy, functional disc repositioning, condylotomy, eminectomy, meniscectomy, or total TMJ replacement (1).
Severely advanced joint pathology, resulting from congenital aplasia, osteoarthritis, ankylosis, tumor, or trauma, with marked loss of function can necessitate a more aggressive approach to include alloplastic total TMJ replacement. Studies within the literature have shown that, outcomes with total TMJ replacement with alloplasts give excellent results (2). An alternative approach to the total TMJ replacement is to utilize a tissue engineering methodology to construct the TMJ to mimic anatomic contours of structures they are replacing and simultaneously, decrease patient morbidity and the average hospital stay. These tissue engineered devices have the advantage of easily adapting to the surgical site and remodeling over time per Wolff’s law (1). Recent biotechnological advances, to include smart biomaterials, 4D printing, scaffold development, and biomechanical/electrical stimuli, are directly relevant to the evolving future of TMJ surgery and complement a tissue engineering approach to TMJ repair and reconstruction. This paper explores how several of these emerging technologies can be incorporated into the future surgical management and treatment of disorders of the TMJ.
Methods
An electronic literature review was conducted via PubMed and Embase on 4/1/2020. The search encompassed articles published through 2020. Thirty-five studies in the English language were identified to provide evidence for new technologies and their influence on the future surgical management and treatment of the TMJ. Topics for the search for the review were chosen based on a lecture by Dr. Stephen E. Feinberg DDS, Phd, titled “TMJ Future: Emerging Technologies”, given in Chicago, IL: TMJ Track: 100th Annual AAOMS Meeting, 2018. Included articles were required to include a topic of at least one of the following: 4D printing, electrospun biomaterials, smart biomaterials, conducting polymers, piezoelectric effect, stem cells, or gene delivery by IA injection. Articles were initially screened by DFW and approved by SEF.
Smart biomaterials
Biomaterials for scaffold fabrication have evolved over time. The first generation consisted of single passive component scaffolds (physical, chemical, biological, or material). The second generation were two component scaffolds, consisting of the combination of nanofibers and small molecules or composite ceramic/polymer scaffolds. This has eventually led to increasingly complex “smart” materials, which are multicomponent scaffolds that interact with their surrounding environment (3,4).
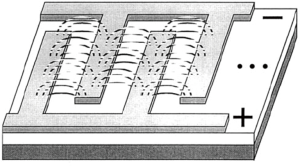
Smart biomaterial scaffolds can be directly implanted in vivo to control the kinetic and temporal release of growth factors to affect the control of drug delivery by utilizing small molecules. Small molecules have the advantages of being selective, potent, water soluble, and cell permeable: properties which are conducive to their use in tissue engineering and regenerative medicine. Nanofibrous scaffolds can be fabricated to mimic native tissue extracellular matrix organization, to the scale of nanometers. These scaffolds are produced by the process of electrospinning, which tailors the fibers to the needs of the specific tissue being repaired (4).
Smart biomaterials that utilize the techniques of electrospinning have a wide variety of therapeutic uses that include the repair of blood vessels, nerve tissues, cartilage, bone defects, and have applications in soft tissue repair. The electrospinning process consists of pumping a polymer solution, via a syringe with a nozzle, to a system. The polymer solution is subjected to a difference in electrical voltage, between the nozzle and a counter electrode, which results in a cone-shaped deformation of the polymer solution. The solvent portion of the polymer solution evaporates during this process, precipitating a solid continuous filament. A multitude of factors control this process, including: environmental parameters, solution properties such as elasticity, viscosity, conductivity, and surface tension, and governing variables such as electrical potential, flow rate, molecular weight of the polymers, and others (5,6) (Figure 1).
Repairing bone defects (caused by trauma, tumors, etc.) is an important application of electospun biomaterials. Emulating the structure of native bone extracellular matrix (ECM) is particularly difficult, because traditional electrospinning materials lack a 3 dimensional (3D) porous structure, which is necessary for nutrient transport, vascular ingrowth, and functional regeneration of the tissue (7). A 3-D printed electrospun nanofibrinous scaffold has recently been developed. The process of preparation consists of nano fiber preparation, homogenization, freeze-drying, and cross-linking. This process results in the formation of fibrous pores, which successfully mimic the architecture of native bone extracellular matrix (ECM) (Figure 1) (6).
Electrospun biomaterials also have crucial importance in the future of vascular tissue engineering. Autologous and allogenic vascular transplantation has the major complication of donor-site morbidity and the procedure is limited by the number of available donors (8). Heparin-loaded poly (L) caprolactone (PLCL) nano fibers prepared with the coaxial electrospinning technique are the preferred biomaterial for vascular tissue engineering. This technique makes use of 4D printing, as the heparin is released over time into the surrounding tissue. Additionally, vascular endothelial growth factors (VEGFs) can also be used in this manner to promote healing and regeneration of the tissue. The combination of heparin and VEGF in this setting also has anticoagulant properties, which are desirable (9). Additionally, the endothelial cell count was significantly increased on VEGF-enhanced scaffolds, indicating its importance in constructing a scaffold for vascular tissue engineering (10).
The application of a biomimetic degradable scaffold is preferred. Biodegradable polymer microspheres coated with an inorganic hydroxyapatite layer allow control over the kinetics of protein, DNA, or small molecule release over time. This enables the controlled kinetic release of a variety of protein, gene, and cell therapies in the field of tissue engineering that mimic the natural process seen within the body (11). Electrospinning affords us the ability to produce a 3D printed biodegradable scaffold with appropriate growth factors to induce bone/cartilage and vasculature for a fabrication of the next generation total joint prosthesis.
4D printing
Bioprinting has been one of the most impactful developments in the field of tissue engineering. The original goal of bioprinting was to create and engineer tissues, held in place by a scaffold, with the intention of replacing damaged or missing tissue. 4D printing makes use of 3D bioprinting technology, with the 4th dimension being material changing morphologically over time in response to various environmental stimuli (such as heat, electricity, and temperature) (12). In 4D printing, there has recently been demonstrated a two-way actuation using a composite of a hydrogel and a one-way shape polymer. The responsive hydrogel swells or shrinks in response to solvent molecules diffusing into a polymer network. The one-way shape memory polymers regulate the time over which the transformation between states occurs. The use of 4D printing in the future could result in advances in minimally invasive arthroscopic surgery using innovative suture material, scaffolds, and drug reservoirs (13).
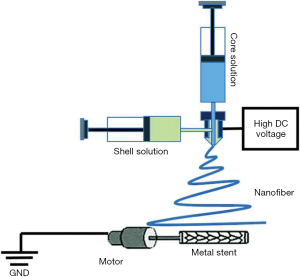
When a material is deformed, the deformed shape can be maintained indefinitely, until the correct stimulus is applied and the material returns to its original shape. This is called the shape memory effect. Surgeons utilizing minimally invasive techniques can achieve significant benefits for their patients, such as: shorter operations, reduced wound healing, and accelerated recovery. However, due to the narrow confines of minimally invasive surgery, shaping tissue in a small space is a challenge. Shape memory polymers could be inserted in one small shape, a stimulus applied intra-operatively, and the desired shape achieved during a minimally-invasive surgical procedure such as in arthroscopy (Figure 2) (14).
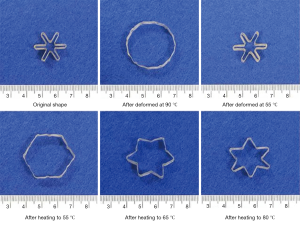
The stimulus that triggers the shape memory polymer to return to its original shape can vary considerably. The simplest example is using heat (in water) to allow a material to return in a one-way shape memory effect. Further, a two-way shape memory effect can be achieved by thermal cycling, when the distortion of the material is kept within a defined limit, creating an internal elastic stress field. A triple-shape memory effect can be achieved by synthesizing the material to have different segments with different properties, each corresponding to a desired shape. The heat generated by the body, and during an intensive operation, is problematic for the application of heat-induced memory polymers. A further application to circumvent the issue with heat-induced shape memory polymers is to either (I) use cooling for shape memory recovery or (II) use a photo-response for shape memory recovery (14). Surgical applications of shape memory polymers are immense. Stents, sutures, clips all have applications in surgery (15).
Flap prefabrication
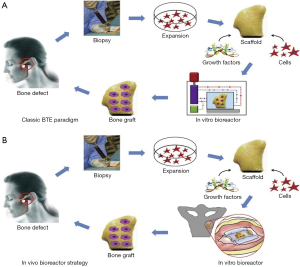
Reconstructing large bony defects via grafts is a challenge due to the complexity of functional and esthetic properties of the bone. The traditional bone tissue engineering (BTE) approach to repair a bony defect would entail the following: obtain a biopsy to select the osteoprogenitor/stem cells that are then expanded/amplified in vitro. A bioscaffold, preferable biodegradable, is fabricated with a 3D printer and seeded with osteoprogenitor/stem cells and growth factors, and cultivated in an in vitro bioreactor. This culminates in a tissue engineered and properly formed bone that can be transplanted to the original bony defect. This is the traditional tissue engineering paradigm to bone reconstruction surgery.
A novel technique has been developed which combines flap prefabrication and using the body as an in situ bioreactor, in place of the in vitro bioreactor used in the traditional approach. This takes advantage of the body’s self-regenerative capacity to regenerate new tissue, and surpasses limitations of an in vitro bioreactor. The in situ flap prefabrication approach allows a vascularized bone flap to be artificially generated in situ to the confines of any shape to match a defect in the bone. Recently, the prefabrication technique has evolved from revascularization and prelamination of flaps to include regeneration, as it is relevant in tissue engineering. This technique has been used successfully in reconstructing critical-sized bone defects in animal models and in humans, and is directly applicable to TMJ surgery (Figure 3) (16).
Biomechanical/electrical stimuli
Tissue engineering has expanded its repertoire to include the use of biophysical manipulation of natural processes to obviate the need for growth factors which are drugs that can complicate the FDA approval process for use in humans. Regenerative biology is traditionally focused on chemical factors and transcriptional networks; however, endogenous ion flows are key epigenetic regulators of cell behavior. These include the roles of endogenous voltage gradients, ion flows, and electric fields in manipulation of the morphogenetic processes as a substitution for growth factors (17).
Biophysical stimulation delivered through an electric field results in a variety of cellular effects: (I) altered movement and concentration of charged cytoplasmic molecules, (II) altering the transmembrane potential via activation of growth-regulating ion transport across the plasma membrane, or (III) cause an electrophoretic accumulation of surface molecules and altered protein conformation at the plasma membrane (18). Alteration of transmembrane potential may occur through direct and capacitive stimulation which results in the opening of voltage-gated calcium (Ca++) ion channels creating an increase in intracellular Ca++. from the endoplasmic reticulum (ER) within the cell. The increase in intracellular Ca++ upregulates calmodulin resulting in a stimulation of a host of other growth factor effects that include: proliferation, increased alkaline phosphatase activity, enhanced extracellular matrix (ECM) deposition, and upregulation of various growth factors, such as, VEGF, BMP-2, and TGF-Beta 1 (19).
The ability to regulate the polarity of the cell membrane utilizing biophysical stimulations offers a unique approach to influence differentiation of stem cells into a variety of cell lineages, i.e, manipulation of a mesenchymal stem cell (MSC) into cartilage or bone (20). This would, theoretically, make it possible to create an osteochondral scaffold, i.e, a joint, by seeding undifferentiated MSCs onto one scaffold (utilizing a conducting polymer; see next section) and varying the biophysical (electrical stimulation) voltage on either sides of the scaffold to direct the cells to either produce bone or cartilage, i.e, formation of an osteochondral graft for joint reconstruction.
Conducting polymers
Novel biocompatible and biodegradable electrically conducting polymers can be used for biosensors and for “smart” tissue engineering scaffolds. A conducting polymer is an organic polymer than can conduct electricity, either by metallic conductivity or by being a semiconductor. Scaffolds composed of conduction polymers can be used for: repair/regeneration of bone and/or cartilage, as primary scaffolds or for directing/influencing progenitor/stem cell activity (see previous paragraph) (3).
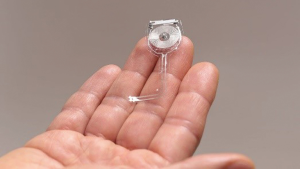
For example, in the event of severe peripheral nerve injury from trauma or surgery, limited motor and sensory functional recovery constitutes a major barrier for full recovery. Direct intraoperative electrical stimulation of an injured nerve has been demonstrated to accelerate functional recovery, which indicates that bioelectric approach could be further indicated for therapy in these patients. A wireless, programmable, bioresorbable electronic system, capable of sustained bioelectrical neuro-regenerative therapy has been developed (21). After a sciatic nerve injury, the device was implanted into the body of the mouse. The cuff was secured to the sciatic nerve with bioresorbable sutures, and the radio frequency harvester unit was implanted subcutaneously. A transmission antenna was used to pass a radio frequency wave to the device, delivering electrical stimulation to the sciatic nerve. Electromyograms (EMGs) taken from the tibias anterior muscle in control mice demonstrated that the device was capable of producing nerve stimulation at levels above threshold. The device delivered pulses of electricity to the damaged peripheral nerves at regular intervals to facilitate functional recovery of the nerve. The device remained in its implanted location for approximately two weeks, before being absorbed and degraded by the body (Figure 4) (21,22).
Conducting polymers can also be used to make site specific scaffolds that consist of interdigitated electrodes. A controlled electric field created from specifically designed degradable scaffolds, such as polyaniline, will be capable of differentiating human bone marrow stromal cells (hBMSC) into both bone-forming osteoblasts and cartilage-forming chondroblasts in different locations on the same scaffold to form an osteochondral graft. The distance and depth between the interdigitated electrodes can control the electrical field strength emitted by the scaffold thus controlling the flow of Ca++ ions (23). The concentraion of Ca++ ions will regulate the membrane potential and affect the cell lineage differentiation (bone vs. cartilage) (24,25) thus potentially creating an osteochondral graft on the same scaffold, i.e, a total joint (Figure 5) (25,26).
Piezoelectric effect and smart biomaterials
The signaling pathways of cells utilize electrical and chemical synapses for communication that can remodel bone and cartilage. One significant challenge with designing biomaterials to take advantage of electrical stimulation for tissue regeneration is the source of electrical stimulation. Traditionally, an external electrode would be required to generate an electrical field to activate the transduction system through a piezoelectric effect. Piezoelectric materials which can be modeled into a scaffold can be polymers, ceramics, or composites. For a material to exhibit piezoelectricity, it must lack a center of symmetry and have certain properties in its crystal lattice structure (27). Piezoelectric materials generate electrical activity when deformed or stressed, circumventing the need for an external supply of electricity. Thus, when a mechanical load is applied to the system, the piezoelectric scaffold acts as a sensitive mechano-electric transduction system; the mechanical stimulus results in the piezoelectric material generating electrical activity, which can be used to enhance tissue regeneration at a target site. Piezoelectric materials have potential applications in power generation, structural health monitoring, and use in the development of tissue engineering devices (28). Following surgical treatment, bone and cartilage are particularly prone to degeneration. Piezoelectric materials show potential particularly as treatment options following autograft, allograft, and chondrocyte implantation, due to the utilization of mechano-electric transduction loads placed on bone and cartilage (29).
This approach can further be assisted by utilizing scaffolds composed of conduction polymers as discussed previously. The stimulatory force could be either mechanical or electrical (piezoelectrical), or by utilizing a wireless, bioresorbable electronic system, capable of sustained bioelectrical external power or a standard micro-battery, as noted above.
Stem cells
One means in which stem cells communicate with their environmental niche is through mechanical forces. These forces can be used to regulate differentiation and self-renewal properties of the cells by interaction with the surrounding extracellular matrix (ECM). Forces can be categorized as internally-generated or externally-applied. The force from a muscle contraction would be internally-generated, as opposed to the force of gravity on the entire body of the organism which is external. Further, these forces have various levels of potency based on the mechanical properties of the specific tissue/cell that is being interacted with. For instance, in a typical stress/strain relationship curve, effective, non-permanent transfer of material can be defined as elastic. Elastic deformation is a property intrinsic to the tissue that would alter the transfer of mechanical forces in stem cell communication. Matrix stiffness and applied forces can be controlled to manipulate stem cell differentiation and renewal. Synthetic materials have been designed that demonstrate these properties. Importantly, the design of these materials can be done such that stem cell self-renewal, proliferation, differentiation, and organ formation can be controlled (30).
Further, externally applied mechanical forces can stimulate stem cells to promote tissue regeneration. This is most evident in the clinical practice of distraction osteogenesis, which utilizes an externally-generated strain on bone, to promote bone formation. Implants in orthopedics serve a similar function, as they are designed to promote strain-mediated effects on bone remodeling and regeneration (31). There is a substantial potential in tissue engineering to expand upon this concept to other tissues. Questions arise as to how strain could impact the regeneration of other tissues, or how externally-applied strain forces affect stem cells in muscle. Future work in this area will likely address these questions.
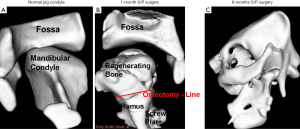
In demonstration of external mechanical forces on the regeneration of the condyle, a condylectomy was performed in Yucatan minipigs. A U-shaped scaffold with biodegradable poly-caprolactone (PCL) screws were placed on the posterior border of the mandibular ramus in the minipigs to replace the condylar head. The minipigs were immediately placed into function to allow for functional remodeling of the TMJ. After 3 months, a neo-condyle was regenerated (1). The importance of this event is to show the potential for remodeling of a tissue engineered condylar-ramus construct to adapt and remodel secondary to Wolff’s law, functional forces, into an anatomically normal appearing condylar head (Figure 6) (1).
In a proof of concept study regarding endogenous cell homing, an anatomically correct bioscaffold was constructed to match the surface morphology of a rabbit’s proximal humeral joint. This was done by laser scanning and reconstruction by computer-aided design (CAD). The articular surface of the proximal humeral condyle was surgically excised and the anatomically correct bioscaffold was placed. Ten rabbits received TGF-B3-infused bioscaffolds, 10 received TGF-B3 free bioscaffolds, and 3 received no replacement for the excised articular surface. TGF-B3 positive bioscaffold rabbits resulted in uniformly distributed chondrocytes in a matrix with collagen type II and significantly greater thickness than the TGF-B3 negative bioscaffold rabbits. This study demonstrated the capabilities of endogenous cell homing. The entire articular surface of the synovial joint was able to regenerate in the TGF-B3 positive bioscaffold rabbits, without the use of cell transplantation (32). This has significant implications because making use of cell homing results in an easy clinical delivery system (relative to cell transplantation), and it maximizes the body’s own regenerative capacity (in situ bioreactor).
Gene delivery to joints by intra-articular injection
In the case of arthritis, which is a chronic, incurable disease, a method to deliver drugs at a sustained, therapeutic concentration can be beneficial to management and treatment of this destructive process. One approach is to deliver cDNAs via intra-articular injection, resulting in a sustained, endogenous production of gene products within the joint over time. There have been clinical trials for rheumatoid arthritis and osteoarthritis using retrovirus vectors for ex vivo gene delivery (33,34). Clinical trials using adeno-associated virus for in vivo delivery is particularly of interest, because it is able to transduce chondrocytes in situ. These trials have promising implications in the treatment of osteoarthritic diseases (Figure 7) (33,35).
A multicenter, double-blind, phase III clinical trial was performed to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients (33). A total of 163 patients with knee osteoarthritis received, intra-articular, either non-transformed or transduced chondrocytes (3:1), retro-virally transduced to overexpress TGF-β1 (TGC). Efficacy was measured over time. TGC was associated with statistically significant improvements in functions and pain in patients with knee osteoarthritis. There was a significantly greater improvement in the International Knee Documentation Committee (IKDC) score, a subjective scale that provides patients with an overall function score, at 52 weeks versus the baseline. Patients treated with TGC showed trends directed toward thicker cartilage and slower growing rates of subchondral bone surface areas (P>0.05). Unexpected adverse events were not observed.
Conclusions
Biotechnology and tissue engineering has advanced substantially in recent years and has the potential to influence surgical management of the TMJ. Major implications on the future of TMJ surgery and treatment will be influenced by the use of electrospun and conducting polymer biomaterials that replace bone defects, the use of biophysical and electrical manipulation as a replacement for growth factors, 4-D printed shape memory polymers for use with minimally invasive surgery, cell homing, gene delivery to joints, and genome editing with CRISPR/Cas. Further investigation in these areas will revolutionize the field of tissue engineering and enhance future applications to regenerative medicine and TMJ surgical treatment in a more non-invasive and effective way.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Frontiers of Oral and Maxillofacial Medicine for the series “Temporomandibular Joint Disorders Diagnosis and Management – What Does the Future Hold?”. The article was sent for external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.org/article/view/10.21037/fomm-2020-tjddm-03/coif). The series “Temporomandibular Joint Disorders Diagnosis and Management – What Does the Future Hold?” was commissioned by the editorial office without any funding or sponsorship. SEF served as the unpaid Guest Editor of the series, and serves as an unpaid editorial board member of Frontiers of Oral and Maxillofacial Medicine from Aug 2019 to Jul 2021. SEF reports other from Tissue Regeneration Systems, outside the submitted work; In addition, SEF has a patent United States Patent 8,275,594 licensed to Depuy/Syntheses, a patent United States Patent 8,478,422 issued, and a patent United States Patent 9,943,410 issued. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Santee W, Aronovich S, Feinberg S. Temporomandibular Joint Total Replacement - TMJ TJR. Switzerland: Springer International Publishing, 2016:281-96.
- Mercuri LG, Ali FA, Woolson R. Outcomes of total alloplastic replacement with periarticular autogenous fat grafting for management of reankylosis of the temporomandibular joint. J Oral Maxillofac Surg 2008;66:1794-803. [Crossref] [PubMed]
- Feinberg SE. “TMJ Future: Emerging Technologies”. Chicago, IL: TMJ Track: 100th Annual AAOMS Meeting, 2018.
- Carbone EJ, Jiang T, Nelson C, et al. Small molecule delivery through nanofibrous scaffolds for musculoskeletal regenerative engineering. Nanomedicine 2014;10:1691-9. [Crossref] [PubMed]
- Braghirolli DI, Steffens D, Pranke P, et al. Electrospinning for regenerative medicine: a review of the main topics. Drug Disc Today 2014;19:743-53. [Crossref] [PubMed]
- Ye K, Kuang H, You Z, et al. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019;11:182. [Crossref] [PubMed]
- Yao Q, Cosme JG, Xu T, et al. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017;115:115-27. [Crossref] [PubMed]
- Chlupác J, Filová E, Bacáková L. Vascular prostheses: 50 years of advancement from synthetic towards tissue engineering and cell therapy. Rozhl Chir 2010;89:85-94. [PubMed]
- Chen X, Wang J, An Q, et al. Electrospun poly(l-lactic acid-co-ɛ-caprolactone) fibers loaded with heparin and vascular endothelial growth factor to improve blood compatibility and endothelial progenitor cell proliferation. Colloids Surf B Biointerfaces 2015;128:106-14. [Crossref] [PubMed]
- Guex AG, Hegemann D, Giraud MN, et al. Covalent immobilisation of VEGF on plasma-coated electrospun scaffolds for tissue engineering applications. Colloids Surf B Biointerfaces 2014;123:724-33. [Crossref] [PubMed]
- Jongpaiboonkit L, Franklin-Ford T, Murphy WL. Mineral-coated polymer microspheres for controlled protein binding and release. ACS Appl Mater Interfaces 2009;21:1960-3.
- Ge Q, Sakhaei AH, Lee H, et al. Multimaterial 4D Printing with Tailorable Shape Memory Polymers. Sci Rep 2016;6:31110. [Crossref] [PubMed]
- de Marco C, Pané S, Nelson BJ. 4D printing and robotics. Sci Robot 2018;3:eaau0449. [PubMed]
- Huang WM. Shape memory polymers (SMPs) – Current Research and Future Applications. Available online: http://azom.com/article.aspx?ArticleID=6038
- Lendlein A, Robert L. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002;296:1673-6. [Crossref] [PubMed]
- Huang RL, Kobayashi E, Liu K, et al. Bone graft prefabrication following the in vivo bioreactor principle. EBioMedicine 2016;12:43-54. [Crossref] [PubMed]
- Levin M. Bioelectric mechanisms in regeneration: unique aspects and future perspectives. Semin Cell Dev Biol 2009;20:543-56. [Crossref] [PubMed]
- Thrivikraman G, Boda SK, Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018;150:60-86. [Crossref] [PubMed]
- Balint R, Cassidy NJ, Cartmell SH. Electrical stimulation: a novel tool for tissue engineering. Tissue Eng Part B Rev 2013;19:48-57. [Crossref] [PubMed]
- Clark CC, Wang W, Brighton CT. Up-regulation of expression of selected genes in human bone cells with specific capacitively coupled electric fields. J Orthop Res 2014;32:894-903. [Crossref] [PubMed]
- Koo J, MacEwan MR, Kang SK, et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat Med 2018;24:1830. [Crossref] [PubMed]
- Fellman M. Biodegradable implant provides electrical stimulation that speeds nerve regeneration. Available online: http://news.northwestern.edu/stories/2018/october/researchs-demonstrate-first-example-of-a-bioresorbable-electronic-medicine/
- Mazlan NS, Ramli MM, Abdullah MM. Interdigitated electrodes as impedance and capacitance biosensors: a review. AIP Conference Proceedings 2017;1885. Available online: https://aip.scitation.org/doi/abs/10.1063/1.5002470
- Xu J, Wang W, Clark CC, et al. Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthritis Cartilage 2009;17:397-405. [Crossref] [PubMed]
- Wang Z, Clark CC, Brighton CT. Up-regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J Bone Joint Surg Am 2006;88:1053-65. [Crossref] [PubMed]
- Epstein AJ, Feinberg SE, Hansford DJ, et al. Electrical Stimulation of Cell and Tissue Growth with Two- and Three- Dimensionally Patterned Electrodes for an inversion entitled: “Electrical Stimulation of Cell and Tissue Growth with Two- and Three-Dimensionally Patterned Electrodes”. United States Patent and Trademark Office: Patent number 8,478,422.
- Jacob J, More N, Kalia K, et al. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regen 2018;38:2. [Crossref] [PubMed]
- Tandon B, Blaker JJ, Cartmell SH. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater 2018;73:1-20. [Crossref] [PubMed]
- More N, Kapusetti G. Piezoelectric material–a promising approach for bone and cartilage regeneration. Medical Hypotheses 2017;108:10-6. [Crossref] [PubMed]
- Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 2017;18:728-42. [Crossref] [PubMed]
- Cilla M, Sara C, Duda GN. Strain shielding inspired re-design of proximal femoral stems for total hip arthroplasty. J Orthop Res 2017;35:2534-44. [Crossref] [PubMed]
- Zhen C, Yo K, Ayesha H, et al. TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis Model Mech 2014;7:83-91. [Crossref] [PubMed]
- Evans CH, Ghivizzani SC, et al. Gene delivery to joints by intra-articular injection. Hum Gene Ther 2018;29:2-14. [Crossref] [PubMed]
- Kim MK, Ha CW, In Y, et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev 2018;29:48-59. [Crossref] [PubMed]
- The Hospital for Sick Children “Joint and Tendon steroid injections using image guidance”. Available online: http://www.aboutkidshealth.ca/Article?contentid=2452&language=English
Cite this article as: Werkman DF, Feinberg SE. Emerging technologies in the surgical management and treatment of the temporomandibular joint: a narrative review. Front Oral Maxillofac Med 2020;2:30.