Cribriform adenocarcinoma of the tongue: case report and review of the literature
Introduction
Since the first report in 1999, cribriform adenocarcinoma of the tongue and minor salivary gland (CATMSG) have been reported in a limited number of papers. In view of its special location, cytology, histological architecture, behavior, and tendency for early nodal metastatic, many scholars tend to classify it as a distinct tumor entity. The tumor needs more recognition and research. This paper analyzes a case from the perspective of clinicians, reviews the previous literature, and summarizes the relevant experience.
Case presentation
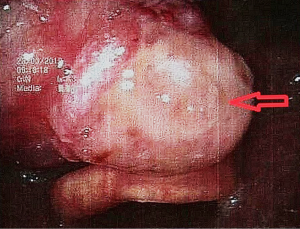
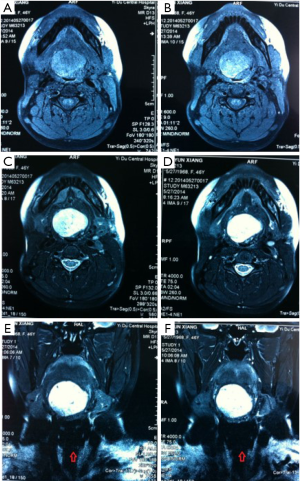
The patient, female, 73 years old, had a foreign body sensation at the base of her left tongue and had a sleep snoring for 2 months. Electronic laryngoscope and MR examination showed that the left tongue base was occupied. The patient’s foreign body sensation was obvious when swallowing saliva, drinking water, and was not obvious when eating, and no breathing difficulties or pain, numbness or bleeding in the tongue was observed. Laryngoscopy showed that the neoplasm was close to the epiglottis near the left side of the tongue. The surface was uneven, and the size was about 1.5×2.0 cm. There was no abnormality in the epiglottis (Figure 1). MR showed a 2.6×2.7×2.7 cm nodule at the base of the left tongue, and T1 and T2 showed equal length signals. After strengthening, the boundary of the lesion was clear, the envelope was obviously strengthened, the internal was unevenly enhanced and the low-signal zone without enhancement was observed. The adjacent oropharyngeal cavity was narrowed (Figure 2).
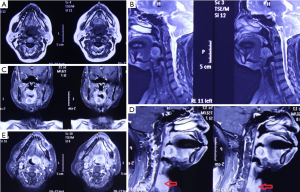
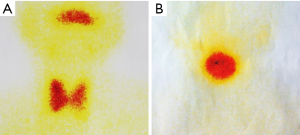
After the patient was admitted to the hospital, a routine preoperative examination was performed to rule out absolute surgical contraindications. Due to the special location of the tumor, although the normal position of the thyroid was visible in the MR scan area (Figure 2D, red arrow), we still performed a thyroid static imaging enhanced computed tomography (ECT) (Figure 3). The results showed that the thyroid was at a normal position, with a regular shape and a normal size. The radioactivity distribution in the two leaves is uneven, and the left leaf radioactivity distribution was slightly sparse, and the radioactive concentration was visible at the left tongue base in the mouth. The thyroid-related serum markers showed that T3, T4, thyroid-stimulating hormone (TSH), and thyroglobulin antibodies were normal except for the elevated thyroid peroxidase antibodies (Table 1). Because the location of the tumor was close to the oropharynx, the lower bottom part of the mouth, the mass was not visible in the mouth when the patient opened the mouth. There were no obvious enlarged lymph nodes in the neck of the patient in clinical examination and MR scan.
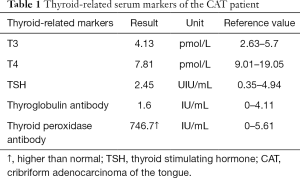
Full table
Because the location of the tumor was located in the oropharynx, full exposure required cutting the mandible and the tongue, and the surgical injury was severe. The patient was old, and the patient and her family were reluctant to adopt a major traumatic surgical plan. After the patient’s informed consent, the surgical plan was determined to be a partial resection of the left tongue mass of the oral cavity. According to the pathological results, if necessary, a second operation or other treatment would be performed.
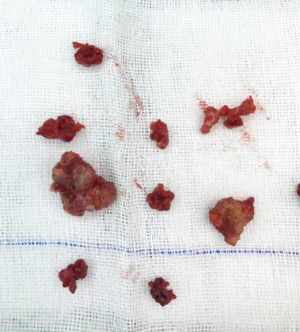
The operation was performed under general anesthesia. As expected before surgery, although the tongue was fully pulled forward under the large opening, the mass was still difficult to be exposed. Also, although the boundary of the tumor was clearly seen as a capsule before surgery, the tumor was found to have no obvious capsule during the resection, which was hard, tough, and brittle. The lower tongue muscle was infiltrated without obvious separation, which was difficult to be removed. Then, the mass was removed part by part. The inside of the tumor and the mass of the tumor adjacent to the muscle tissue of the tongue was of a fish-bone-like sensation. A curette was used to scrape the resected wound thoroughly until the palpation of the tongue muscle tissue was free of fish-bone-like sensation. After the wound stopped bleeding, the absorbed suture was used. The excised mass is shown in Figure 4, with a total volume of about 3×3×1.5 cm. Pathology: tongue cribriform adenocarcinoma with calcification and ossification, CKAE1/AE3(+), CK8/18(+), CK7(+), 34βE12(+), vimentin partially (+), p63 partially (+), P53(+)S accounted for 5%, Ki-67(+)S accounted for 1%; TTF-1(–), thyroglobulin(–), CK5/6(–), S-100(–), glycogen PAS(–).
The patient recovered well after surgery and no adverse complications occurred. Because there was no evidence of cervical lymph node metastasis, the patient only received localized adjuvant radiotherapy in the lesion area. The patient was followed up for 10 months, and the condition was stable without recurrence and metastasis.
Discussion
Cribriform adenocarcinoma of the tongue (CAT) is the tumor originally described in 1999 by Michal et al. (1). In the original cases, all 8 neoplasms were located in the tongue. After the original paper was published, identical tumors located outside the tongue, including the soft palate, retromolar buccal mucosa, palatine tonsil and lip were received. CAT was renamed as CATMSG by Michal’s group (2). According to the 2017 WHO Classification of Head and Neck Tumors, CATMSG is introduced for the first time as the terminology “cribriform variant of polymorphous adenocarcinoma”. At the same time, the new classification renamed polymorphous low-grade adenocarcinoma (PLGA) as polymorphous adenocarcinoma (PAC), and classified CATMSG within the histological spectrum of PAC. However, many scholars have reported that CATMSG is a distinct neoplasm, it has special biological behavior: more than half of the patients have lymph node metastasis at the time of presentation, even a neck mass can be the initial symptom. In terms of genetic testing, most PACs harbor recurrent PRKD1 E710D hotspot mutations, whereas CATMSGs display rearrangements PRKD1/2/3. So, whether CATMSG should be considered separately from PLGA is still controversial.
CAT occurs exclusively in the tongue, especially in the base of the tongue, and has a nucleus very similar to that of papillary adenocarcinoma of the thyroid gland. These result in a presumption that cribriform adenocarcinoma may have an origin in the lingual thyroglossal duct anlage. In the case, the tumor was located at the base of the tongue. Interestingly, static ECT imaging of the thyroid showed a weakening of the left thyroid gland, and the tumor was located just to the left of the base of the tongue and radioactively concentrated (Figure 3A). Because similar tests have not been reported in previous literature, whether this case indicates that the formation of the tumor at this position is related to the residual thyroid gland tube should be further investigated.
Pathologically, pathologists have summarized many features that can be used to diagnose CATMSG. Grossly, the tumors are unencapsulated, white to grey in color, and hard in consistency with no areas of necrosis and hemorrhage. Although it has invasive margin and infiltrates the muscular layer of the tongue or adjacent tissues, the tumor is always covered by an intact mucosal epithelium. The tumor is composed predominantly of cribriform to microcribriform and solid structures. The peripheral layer of solid tumor nests is a layer of palisaded pattern cells. The cribriform to microcribriform structure consists of irregular tubules which are formed by a single cell layer. The nuclei often overlap one another, and are pale, optically clear and vesicular with a ground glass appearance. Cellular atypia is mild, and mitotic figures are rare. Our case was the same as what described above.
Immunohistochemically, previous studies have reported that cytokeratin markers (AE1-3, CK7, CK8, CK18) as well as vimentin, s-100 protein are positive, CK5/6, p53 and p63 demonstrate variable percentages of positive. The Ki-67 proliferation index is low. More importantly, TTF-1 and thyroglobulin are negative in all the tumors. The immunohistochemical results of our cases were consistent with the majority of the current literature reports (Figure 5), that only s-100 protein andCK5/6 were negative, indicating that there was no differentiation of s-100 protein and CK5/6 in the tumor cells, reflecting the heterogeneity.
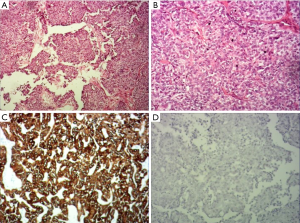
In terms of diagnosis, the previous literature emphasized the difference in the rate of lymph node metastasis between PLGA and CATMSG, and clarified the difference in pathological manifestations between the two. Pathological criteria for differential diagnosis have been gradually developed in the professional field, which is not repeated here. For clinicians, when a specific site tumor (such as the base of the tongue or the bottom of the mouth) is encountered, enlargement of lymph nodes in the neck occurs early in the detection of tumor or after the tumor is removed for a period of time (sometimes several years), especially when the pathology report showed the primary tumor was very rare, and the type of disease was low-grade salivary gland tumors, the possibility of CATMSG should be considered. The pathological sections should be reviewed with the pathologist in time to avoid misdiagnosis and mistreatment as reported by Laco et al. (3).
For the surgeon, because of the special predilection site of CAT, it should be distinguished from the lingual thyroid or ectopic thyroid. The ectopic thyroid is a developmental disease caused by the partial or entire thyroid not falling and staying in the foramen cecum during embryonic development. 90% of the ectopic thyroid is located in the base of the tongue and the area of the epiglottis. The site of the disease is the same as CAT, and it is often manifested as a foreign body sensation at the base of the tongue, swallowing discomfort, affecting speech and eating. Unlike CAT, which is based on surgical treatment, the lingual thyroid is mainly treated with medicine because it may cause adverse consequences to the patient after removal of the thyroid gland. The author has consulted a patient with ectopic thyroid, which is a 45-year-old woman. The main complaint is swallowing discomfort, and the speech is vague for half a year. The MR is shown in Figure 6. It can be seen that the border of the tongue is similar to the CAT. The T1 is of equal signal, the T2 is of high signal, and the thyroid (Figure 6C, red arrow) is not seen in the middle of the neck. Serological markers of thyroid showed that T3 and T4 were significantly lower and TSH was significantly increased (Table 2). It was diagnosed as an ectopic thyroid by the radionuclide scan ECT, showing significant radioactive concentration in the oral cavity and no radiation concentration in the normal thyroid gland (Figure 3B). The patient adopted our recommendation for conservative drug treatment in the endocrinology department. After 5 years, the patient was stable, the serological index became normal, the tumor was reduced, and the symptoms such as swallowing were alleviated. Given the same location of CAT and lingual thyroid, similar clinical symptoms but completely different treatments, the doctors should pay special attention to the identification of the two. Thyroid color Doppler can easily show the presence or absence of thyroid in the normal position. CT and MR can visualize the image, and thyroid radionuclide imaging can reflect the position and function at the same time, which confirms the diagnosis.
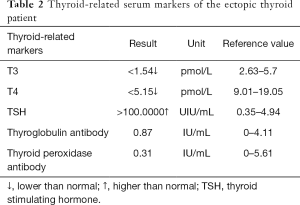
Full table
As for prognosis, CATMSG is locally invasive and has a tendency for early regional lymph node metastasis. By 2019, a total of 69 cases that including follow-up information were reported in the literature, 41 cases were located in the tongue (usually the base). The age of the patients ranged from 21 to 85 (mean 56.8) years. The cervical lymph node metastases were present at the time of diagnosis in 35/69 of the patients. The follow-up time range 2 to 217 months. The distant metastatic rate is very low and only 2 reported patients have died due to the disease (See Table 3 for more information). In general, CATMSG is a low grade malignant, non-fatal tumor in most cases. Unlike reports from the literature that early cervical lymph nodes are both enlarged and metastatic, no clinical evidence of cervical lymph node metastasis was observed in our cases. Pathological examination demonstrated the calcification and ossification of the tumor. We speculated that CAT with calcification and ossification may have a milder biological behavior and a lower tendency to metastasize. Of course, a longer follow-up observation is required.
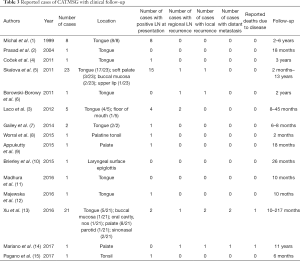
Full table
Typically, CATMSG is treated by surgical excision often accompanied by neck lymph node dissection. For the tumor that occurs in the base of the tongue, in order to fully expose the surgical field, it is necessary to disconnect the soft and hard tissues of the lower jaw, as well as to perform (bilateral) lymph node dissection of the neck. Thus, the surgical trauma is extremely large, and the postoperative recovery time is long, and it is prone to adverse complications. Our patient adopted a conservative strategy of local resection combined with postoperative radiotherapy. That is for two reasons. First, patients and their families were not willing to undergo a wide range of traumatic surgery. Second, the existing literature showed that CAT has a low degree of malignancy. Local excision with radiotherapy can also achieve good therapeutic effects in most cases. Even if cervical lymph node metastasis occurs, the survival period is mostly not affected, i.e., the patient has a very low probability of death due to the disease. The only two death cases were both lesions that occurred in the soft palate. One was a 25-year-old female at diagnosis with metastases to the bone and lung at presentation (13). The time from presentation to her death was 9.3 years. The other was a 78-year-old male who gradually developed 5 regional metastases over 11 years of follow-up (14). We believe that the treatment of CAT should be comprehensive. Especially for elderly patients, it should be personalized to reduce the means of trauma, and to better recover and ensure the quality of life. Worrall et al. described a previously unreported use of transoral robotic surgery (TORS) for the local resection of CATMSG in the palatine tonsil (8). By using superior visualization technology, surgical resection can obtain excellent local control with minimal trauma in comparison to conventional transoral surgical approaches. We believe that endoscopy is a worthwhile choice to visualize the tumors that occur in the oropharynx and base of the tongue and is also the trend of minimally invasive concepts.
In summary, we discussed in detail the situation of the CATMSG patient from the clinical manifestations, examinations, and pathology, combined with our own experience and literature review, and summarized the clinical diagnosis and treatment approaches for peer reference. The tumors at the base of the tongue should be differentiated from the ectopic thyroid. If the pathological diagnosis shows that it is a very rare disease type or a low-grade salivary gland tumor (PLGA), the doctors should pay attention to the possibility of CATMSG and to the cervical lymph node and review the pathological examination if necessary. For treatment, if necessary, the lesion resection + selective or radical neck dissection should be preferred, and radiotherapy can be adopted according to the postoperative pathology of cervical lymph node. For elder patients, the patients without clinical evidence of cervical lymph node metastasis, local excision combined with radiotherapy may be used, and the situation of the cervical lymph nodes should be closely followed up. For the tumors located in the back of the mouth, especially in the tongue base and the epiglottic valley, the exposure operation is difficult. In order to avoid the extra trauma and complications caused by the conventional approach, the endoscopically assisted or surgical robot-assisted visualized operation can achieve the purpose of minimally invasive surgery. In addition, the patient should be followed up to observe long-term curative effect.
Acknowledgments
We thank the patients and their families for participating in our study. We also thank the colleagues of Department of Pathology, Central Hospital of Zibo, Shandong, China, who helped us with this study.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.com/article/view/10.21037/fomm.2020.01.01/coif). The authors have no conflicts of interest to declare
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Michal M, Skálová A, Simpson RH, et al. Cribriform adenocarcinoma of the tongue: a hitherto unrecognized type of adenocarcinoma characteristically occurring in the tongue. Histopathology 1999;35:495-501. [Crossref] [PubMed]
- Prasad KC, Kaniyur V, Pai RR, et al. Pedunculated cribriform adenocarcinoma of the base of the tongue. Ear Nose Throat J 2004;83:62-4. [Crossref] [PubMed]
- Laco J, Kamarádová K, Vítková P, et al. Cribriform adenocarcinoma of minor salivary glands may express galectin-3, cytokeratin 19, and HBME-1 and contains polymorphisms of RET and H-RAS proto-oncogenes. Virchows Arch 2012;461:531-40. [Crossref] [PubMed]
- Coček A, Hronková K, Voldánová J, et al. Cribriform adenocarcinoma of the base of the tongue and low-grade, polymorphic adenocarcinomas of the salivary glands. Oncol Lett 2011;2:135-8. [Crossref] [PubMed]
- Skalova A, Sima R, Kaspirkova-Nemcova J, et al. Cribriform adenocarcinoma of minor salivary gland origin principally affecting the tongue: characterization of new entity. Am J Surg Pathol 2011;35:1168-76. [Crossref] [PubMed]
- Borowski-Borowy P, Dyduch G, Papla B, et al. Cribriform adenocarcinoma of the tongue. Pol J Pathol 2011;62:168-71. [PubMed]
- Gailey MP, Bayon R, Robinson RA. Cribriform adenocarcinoma of minor salivary gland: a report of two cases with an emphasis on cytology. Diagn Cytopathol 2014;42:1085-90. [Crossref] [PubMed]
- Worrall DM, Brant JA, Chai RL, et al. Cribriform adenocarcinoma of the tongue and minor salivary gland: transoral robotic surgical resection. ORL J Otorhinolaryngol Relat Spec 2015;77:87-92. [Crossref] [PubMed]
- Appukutty SJ, Di Palma S, Daborn L, et al. Cribriform adenocarcinoma of minor salivary glands. Diagn Histopathol 2015;21:380-2. [Crossref]
- Brierley D, Green D, Speight PM. Cribriform adenocarcinoma of the minor salivary glands arising in the epiglottis--a previously undocumented occurrence. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:e174-6. [Crossref] [PubMed]
- Madhura MG, Kumar BV, Suma S, et al. Cribriform adenocarcinoma of minor salivary gland: a mimic of polymorphous low-grade adenocarcinoma. J Oral Maxillofac Pathol 2016;20:536-9. [Crossref] [PubMed]
- Majewska H, Skálová A, Weinreb I, et al. Giant cribriform adenocarcinoma of the tongue showing PRKD3 rearrangement. Pol J Pathol 2016;67:84-90. [Crossref] [PubMed]
- Xu B, Aneja A, Ghossein R, et al. Predictors of outcome in the phenotypic spectrum of polymorphous low-grade adenocarcinoma (PLGA) and cribriform adenocarcinoma of salivary gland (CASG): a retrospective study of 69 patients. Am J Surg Pathol 2016;40:1526-37. [Crossref] [PubMed]
- Mariano FV, Varanda RF, Schultz L, et al. Cribriform adenocarcinoma of the soft palate with multiple lymph node metastasis and long-term follow-up. Oral Surg Oral Med Oral Pathol Oral Radiol 2017;123:e117-22. [Crossref] [PubMed]
- Pagano A, Dennis K. Cribriform adenocarcinoma of the minor salivary gland arising in the tonsil with metastasis to a cervical lymph node: a case report with description of fine needle aspiration cytology. Diagn Cytopathol 2017;45:468-71. [Crossref] [PubMed]
Cite this article as: Xu C, Yu L, Song Y, Che Z. Cribriform adenocarcinoma of the tongue: case report and review of the literature. Front Oral Maxillofac Med 2020;2:3.