
Chronic mucocutaneous candidiasis with STAT1 gain-of-function variant: a case report and review of the literature
Introduction
Chronic mucocutaneous candidiasis (CMC) is a clinically heterogeneous disease characterized by susceptibility to chronic or recurrent superficial Candida infection of the skin, nails and mucous membranes (1). There are various causes of recurrent oral Candida infection, such as human immunodeficiency virus infection and use of glucocorticoids or antibiotics. However, rare primary gene mutations can also cause Candida infection. Studies have demonstrated that mutations in gene signal transducer and activator of transcription 1 (STAT1), autoimmune regulator (AIRE), caspase recruitment domain-containing protein 9 (CARD9) and Dectin-1 may be associated with CMC (2). In this study, we present a case of CMC due to a gain-of-function (GOF)-STAT1 mutation and briefly review the CMC related to mutated GOF-STAT1 by collecting data from databases, including Web of Science, Science Direct, PubMed and Google Scholar.
Case presentation
A 14-year-old boy complaining of persistent white membranes in the oral cavity for 14 years was referred to the oral clinic of our hospital for diagnosis and treatment. He had been using sodium bicarbonate mouthwash for many years with a poor response. The patient experienced recurrent oral ulcers in early infancy and repeated keratitis at the age of 2 years. He was physically poor and often required sick leave from his study. There is no family history of illness.
On physical examination, the patient had erythematous patches in the center of his face and nose. There was a white pseudomembrane covering his entire oral cavity, including the palate, tongue and buccal mucosa. The nail on his right forefinger was hyperkeratotic with white discoloration.
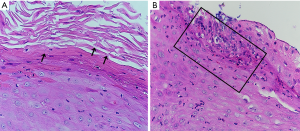
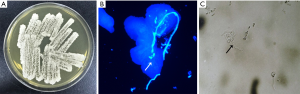
Biopsy of the left side of tongue showed proliferative candidiasis with mild epithelial hyperplasia (Figure 1). Fungal culture of the oral white pseudomembrane, fungal fluorescent staining of the right forefinger and KOH microscopic examination of the skin all revealed Candida-infection (Figure 2). A blood sample was obtained and complete blood counts, and various biochemical and immunological parameters were measured. The complete blood counts were grossly normal apart from a high absolute monocyte count (0.65×109/L, normal range, 0.1–0.6×109/L) and a low percentage of neutrophil nuclear cells (37.1%, normal range, 40–75%). The absolute neutrophil counts were 1.9×109/L, which was within the normal range. Immunoglobulin (Ig) levels (IgM/IgA/IgE) were all within normal limits with the exception of high IgG (17.2 g/L, normal range, 7–16 g/L). An examination of lymphocyte subsets showed low CD4 lymphocytes (21.32%, normal range, 31–60%), high CD8 lymphocytes (42.44%, normal range, 13–41%), and thus a low CD4/CD8 lymphocyte ratio (0.51, normal range, 0.9–3.6).
In view of the patient’s CMC, autoimmunity and combined immunodeficiency, we performed whole-exome sequencing (WES) with informed consent from his parents to identify genetic causes of the disease. By screening CMC-related genes, we identified a missense variant (NM_007315:c.821G>A, p.R274Q) in exon 10 of the STAT1 gene (Figure 3A). The variant c.821G>A (nucleotide No. 821 from guanine to adenine in the coding region) in STAT1 resulted in a change of amino acid at p.R274Q (amino acid No. 274 from arginine to glutamine). According to the guidelines of the American College of Medical Genetics and Genomics and the Association of Molecular Pathology (3), this p.R274Q variant can be categorized as pathogenic based on the following factors: PS1, the pathogenicity of this variant for CMC has been reported in the Human Gene Mutation Database. PM2, the frequency of this variant in the normal population database is low. PP3, bioinformatics protein function prediction software SIFT, PolyPhen_2 and REVEL all predicted this variant to be harmful. Sanger sequencing showed the heterozygous STAT1 variant in the patient, whereas his parents did not have this variant, indicating that the variant was a spontaneous mutation (Figure 3B). The final diagnosis was CMC with STAT1-GOF variant.

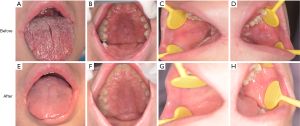
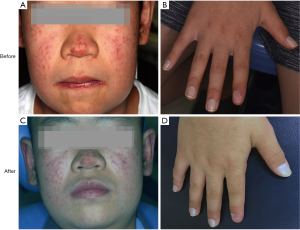
The patient was treated with oral fluconazole 100 mg/day and ketoconazole ointment was applied to the skin lesions every day. His oral symptoms completely disappeared and the result of fungal culture for the oral swab was negative after 3 weeks of treatment (before treatment: Figure 4A,B,C,D; after treatment: Figure 4E,F,G,H). No recurrence was observed 3 months after discontinuation of fluconazole administration. There was also partial regression of the skin lesions, but this was not as obvious as that of the oral cavity. However, no change in his fingernail was observed after treatment (before treatment: Figure 5A,B; after treatment: Figure 5C,D). The patient was arranged regularly for a follow-up every three months. At each visit, the fungal culture was operated and all the results were negative. During one-year follow-up, the condition was stable.
Discussion
We described a young patient with a GOF-STAT1 variant. The majority of GOF-STAT1 mutations affect the coiled-coil domain or DNA-binding domain (4). STAT1 mutations are phosphorylated, and then increase responses to STAT1-dependant cytokines, such as interferon α/β/γ and interleukin (IL)-27. Finally, these mutations impair the development of IL-17-producing T cells (5). IL-17 has recently been identified as a necessary cytokine in human mucocutaneous defense against Candida infection (6).
Major symptoms of CMC include scaling and hyperkeratosis of the skin, erythema of periungual skin, oral thrush, deformation and edema of the fingernails due to persistent Candida infections (7). Toubiana et al. studied patients with GOF-STAT1 mutations who had heterozygous GOF-STAT1 and an unexpected broad clinical phenotype (8). In addition to CMC, these patients were prone to bacterial (74%) infections, mainly due to Staphylococcus aureus (36%), and viral (38%) infections, mainly due to Herpesviridae (83%). Thirty-seven percent of these patients had autoimmune manifestations, including hypothyroidism (22%), type 1 diabetes (4%), cytopenia (4%) and systemic lupus erythematosus (2%). Alarmingly, patients with CMC had a greater risk of developing both oral and esophageal cancer (8-10), and the patients were often much younger than those without GOF-STAT1 mutations. Patients with GOF-STAT1 mutation should pay close attention to the disease and have regular physical examinations and follow-up visits in order to ensure early detection and treatment of complications.
The prognosis of CMC patients is poor. The main treatment for patients with CMC is systemic and topical antifungals. Fluconazole is well tolerated and easy to administer. However, it is harmful to the liver and has the potential for development of drug-resistant strains (11). Drug-resistant Candida strains are a common concern. Additional antibiotic prophylaxis and intravenous immunoglobulin can also be considered in patients with recurrent infections (12). In addition to conventional treatments, studies on new immunological therapies have also made progress. Shahar et al. reported that a CMC patient with GOF-STAT1 had a beneficial clinical response following treatment with granulocyte macrophage colony stimulating factor (GM-CSF) (13). However, a subsequent study reported failure of this treatment (14). Leiding et al. used hematopoietic stem cell transplantation (HSCT) in 15 patients with GOF-STAT1 and made the conclusion that despite having curative potential, only 40% overall post-transplant survival was achieved (15). At present, new insights into hypermorphic STAT1 mutations have identified promising therapeutic targets in the dysregulated STAT1/STAT3/IL-17 pathways (16), such as Janus kinases (JAKs). Bloomfield, Higgins E and Forbes LR all successfully treated CMC patient with STAT1-GOF mutation with the JAK1/JAK2 inhibitor ruxolitinib (17-19). This therapy can be a viable option for treatment for refractory CMC. For diseases caused by gene mutations, the main treatments should target to the symptoms, with the aim to prevent the development of complications and the ultimate goal of cure.
Acknowledgments
Funding: Key Specialty Construction Project {[2013]544}, National Key R&D Program of China (2017YFC0840100, 2017YFC0840110) and Shanghai’s top priority clinical medicine center (2017ZZ01011). Clinical Research Project of Multi-Disciplinary Team, Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine [201701006].
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.org/article/view/10.21037/fomm.2019.09.03/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s parent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Glocker E, Grimbacher B. Chronic mucocutaneous candidiasis and congenital susceptibility to Candida. Curr Opin Allergy Clin Immunol 2010;10:542-50. [Crossref] [PubMed]
- van de Veerdonk FL, Plantinga TS, Hoischen A, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 2011;365:54-61. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Sampaio EP, Hsu AP, Pechacek J, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol 2013;131:1624-34. [Crossref] [PubMed]
- Green L, Dolen WK. Chronic Candidiasis in Children. Curr Allergy Asthma Rep 2017;17:31. [Crossref] [PubMed]
- Puel A, Cypowyj S, Marodi L, et al. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol 2012;12:616-22. [Crossref] [PubMed]
- Khosravi AR, Mansouri P, Saffarian Z, et al. Chronic mucocutaneous candidiasis, a case study and literature review. J Mycol Med 2018;28:206-10. [Crossref] [PubMed]
- Toubiana J, Okada S, Hiller J, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood 2016;127:3154-64. [Crossref] [PubMed]
- Koo S, Kejariwal D, Al-Shehri T, et al. Oesophageal candidiasis and squamous cell cancer in patients with gain-of-function STAT1 gene mutation. United European Gastroenterol J 2017;5:625-31. [Crossref] [PubMed]
- Rosa DD, Pasqualotto AC, Denning DW. Chronic mucocutaneous candidiasis and oesophageal cancer. Med Mycol 2008;46:85-91. [Crossref] [PubMed]
- Popp C, Hampe IAI, Hertlein T, et al. Competitive Fitness of Fluconazole-Resistant Clinical Candida albicans Strains. Antimicrob Agents Chemother 2017; [Crossref] [PubMed]
- Li PH, Lee PP, Fung SL, et al. Chronic mucocutaneous candidiasis-more than just skin deep. Hong Kong Med J 2018;24:423-5. [Crossref] [PubMed]
- Shahar E, Kriboy N, Pollack S. White cell enhancement in the treatment of severe candidosis. Lancet 1995;346:974-5. [Crossref] [PubMed]
- van de Veerdonk FL, Koenen HJ, van der Velden WJ, et al. Immunotherapy with G-CSF in patients with chronic mucocutaneous candidiasis. Immunol Lett 2015;167:54-6. [Crossref] [PubMed]
- Leiding JW, Okada S, Hagin D, et al. Hematopoietic stem cell transplantation in patients with gain-of-function signal transducer and activator of transcription 1 mutations. J Allergy Clin Immunol 2018;141:704-17.e5. [Crossref] [PubMed]
- Lorenzini T, Dotta L, Giacomelli M, et al. STAT mutations as program switchers: turning primary immunodeficiencies into autoimmune diseases. J Leukoc Biol 2017;101:29-38. [Crossref] [PubMed]
- Bloomfield M, Kanderova V, Parackova Z, et al. Utility of Ruxolitinib in a Child with Chronic Mucocutaneous Candidiasis Caused by a Novel STAT1 Gain-of-Function Mutation. J Clin Immunol 2018;38:589-601. [Crossref] [PubMed]
- Higgins E, Al Shehri T, McAleer MA, et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol 2015;135:551-3. [Crossref] [PubMed]
- Forbes LR, Vogel TP, Cooper MA, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol 2018;142:1665-9. [Crossref] [PubMed]
Cite this article as: Yao YL, Shen XM, Shi LJ, Li CX, Wu L. Chronic mucocutaneous candidiasis with STAT1 gain-of-function variant: a case report and review of the literature. Front Oral Maxillofac Med 2019;1:8.

