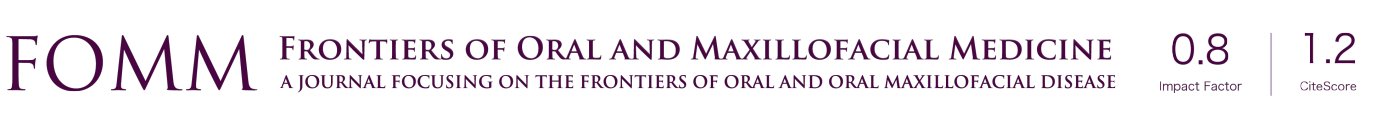Processed nerve allografts in reconstructive microneurosurgery after ablative head and neck surgery: an overview
Introduction
Reconstructive surgery in the head and neck region has significantly advanced in the last decade, with the popularization of virtual surgical planning (VSP), improvement in free tissue transfer techniques, as well as customizable patient-specific implants (PSI). The next frontier of functional reconstruction that has gained significant attention in the last several years is nerve reconstruction and regeneration. Several nerves in the head and neck are placed at risk during surgical intervention, both motor and sensory, resulting in significant postoperative deficits. One of the motor nerves of greatest concern owing to its significant contribution to facial function is the facial nerve (cranial nerve VII), with damage resulting in clinically significant issues with facial animation, as well as problems eating, speaking, and ocular globe protection. Other motor nerves of concern in head and neck procedures include the spinal accessory (cranial nerve XI—Figure 1) and the hypoglossal nerve (cranial nerve XII), however these are mostly encountered during neck dissection procedures, and less so as a result of ablation of primary head and neck tumors. The most significant sensory innervation to consider are branches of all three divisions of cranial nerve V, including supraorbital, supratrochlear nerves, as well as the infraorbital nerve (Figure 2). In addition, the greater auricular (Figure 3) and auriculotemporal nerves are fairly superficial and can be injured in surgeries involving the neck and pre-auricular approaches. Of all the branches of the trigeminal system, however, lingual nerve (LN) and inferior alveolar nerve (IAN) dysfunction lead to the most significant morbidity and impairment, and will be the focus of this article. With the advent of cone-beam computed tomography (CBCT), magnetic resonance neurography (MRN), endoscopic-assisted surgery, and allogenic nerve grafts, the techniques for diagnosis and management of patients with nerve injuries has seen significant improvement. Nerve reconstruction after head and neck ablative surgery represents a particular area where patients can benefit from the restoration of sensation to optimize return of function and quality of life, whether in patients with benign or malignant tumors, osteomyelitis, osteoradionecrosis (ORN), or medication-related osteonecrosis of the jaws (MRONJ).
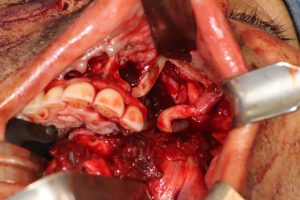
Anatomy
Cranial nerve V, also known as the trigeminal nerve, represents the major sensory innervation of the maxillofacial complex, and is divided into three components (V1, V2, and V3), innervating the upper, middle, and lower thirds of the face, respectively. Sensory stimuli from these branches terminate in the trigeminal ganglion, also known as the Semilunar or Gasserian ganglion, located within Meckel’s cave in the posteromedial aspect of the middle cranial fossa. The ophthalmic branch (V1) is responsible for sensation above the palpebral fissure, as well as the eye and portions of the nasal cavity. The maxillary division (V2) receives sensory input from the nasal cavity, paranasal sinuses, maxillary teeth, and midfacial skin. The mandibular branch (V3) is unique in that it contains both visceral efferent as well as somatic afferent fibers, with the former controlling the muscles of mastication, anterior digastric, mylohyoid, and velar muscles, and the latter divided into the auriculotemporal, lingual, inferior alveolar, and long buccal nerves, providing sensory input from the oral cavity mucosa, anterior two-thirds of the tongue, mandibular teeth, and lower facial skin. Shortly after the LN divides off from the main V3 trunk, chorda tympani, a branch of cranial nerve VII, joins it along its pathway to carry parasympathetic innervation to the submandibular and sublingual glands, as well as taste sensation from the anterior two-thirds of the tongue. The LN then enters the oral cavity between the attachments of the superior pharyngeal constrictor and mylohyoid muscles, continues within the floor of the mouth lateral to the hyoglossus muscle, crosses the submandibular duct, and terminates in the tongue. The LN is a poly-fascicular nerve, ranging anywhere from 9 to 18 fascicles, and averaging 3.2 mm in diameter (1). The IAN descends from the cranial base between the lateral and medial pterygoid muscles, and proceeds to the medial aspect of the ramus of the mandible to enter the mandible at the lingula. The nerve terminates as the mental nerve exiting the mandible at the level of the premolars, and supplies sensation to the ipsilateral lip and chin. The IAN is also a poly-fascicular nerve, containing anywhere between 12 to 21 fascicles, with an average diameter of 2.4 mm (2).
Surgical nerve defects and management
Treatment of head and neck pathology can sometimes necessitate the sacrifice of certain vital structures, including nerves, in the effort to obtain adequate surgical margins and to perform an oncologically safe procedure. Moreover, in the case of malignancies, perineural invasion can become a factor in the decision making process for nerve resection with the goal of providing a curative surgery. In some situations, however, in particular certain benign pathologies, nerves that are in proximity, adjacent, or even within the diseased region, can be saved without compromising the oncologic safety of the procedure.
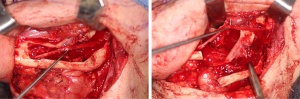
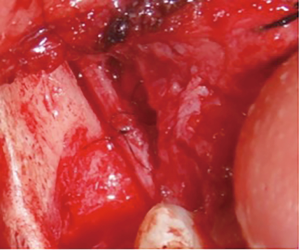
There are several methods available in management of nerves that are involved with, or in close proximity of, benign or malignant pathology. One option is to resect the tumor and sacrifice the nerve, without nerve reconstruction; this technique, of course, commits the patient to complete sensory loss at the dermatome supplied by that particular nerve. The second involves resection of the tumor and nerve, with delayed reconstruction, which carries the risk of scar development around the residual nerve stumps as well as difficulty in locating the proximal stump, making reconstruction more challenging and less predictable in the future. The final option is resection with immediate reconstruction of the nerve; generally speaking, this method provides the best chance of functional neurosensory recovery (FNSR) without compromising the oncologic safety of the procedure. Other techniques have been proposed, including the nerve “pull through” technique (in which the IAN is pulled out of the mandible through the lingula proximally—Figure 4) and the nerve preservation technique (in which a lateral cortical window is created in the bone and the nerve is separated from the site of the tumor in order to preserve it during resection—Figure 5). These techniques have their place in the armamentarium of the surgeon, however this depends on the type of pathology, with the primary goal being to provide an oncologically safe resection to maximize the patient’s survival while minimizing morbidity. Even in the case of certain benign pathology, as in cases of certain ameloblastomas, studies have shown evidence of perineural invasion and recommendations have been made against nerve preservation (3). Loss of function from sacrifice of the LN or the IAN is a cause of significant morbidity for patients postoperatively, resulting in loss of sensation of the anterior two-thirds of the tongue, altered taste, loss of sensation of the ipsilateral portion of the lower lip, chin, and the mandibular dentition, trouble chewing, swallowing, constant trauma to the tongue and/or lip secondary to lack of mechanoreception and nociception, poor manipulation of food bolus, drooling, impaired facial animation, trouble speaking, dysesthesia from neuroma formation, impaired digestion and acid reflux (4). These can lead to decreased enjoyment of food and eating with its associated psychological and physical consequences (depression, weight changes, etc.) (5).
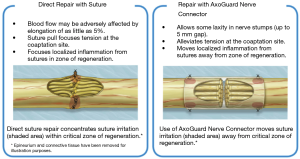
Nerve reconstruction at any stage can be outlined in three different methods: direct suture neurorraphy, conduit-assisted repair, and repair with interpositional grafting. The decision between which method to use depends on several key factors. First and foremost, the coaptation must be absolutely tensionless, as this has been shown time and time again to interfere with axonal growth (6) while increasing scar tissue formation, and it is important not to have more than 2 mm gap between nerve endings, though studies have shown good success in primary repair of LNs with up to 4 mm gaps (7). One study showed that when a tension force greater than 25 g exists across the coaptation, success rates begin to decrease (8), however having said this, resection of nonviable or damaged nerve tissue and obtaining adequate surgical margins should supersede preservation of nerve length for tensionless repair. Direct neurorrhaphy does demonstrate significant success rates if done in select cases with proper surgical technique. Bagheri et al. (9) observed an 88.9% recovery rate in those patients treated with direct neurorrhaphy (16 of 18 total patients), though in a study by Mozsary and colleagues (10), only 12 of the 18 LN injury patients treated with direct repair had FNSR and Susarla found that these patients had a higher rate of neuroma formation (11). In situations where direct neurorrhaphy is not possible or that tension is inevitable, a tension-free repair can be accomplished with a conduit-assisted repair, to allow for axonal guidance across the gap and preventing axonal escape, as well as decrease inflammation in the region of nerve regeneration (Figure 6). Historically, several types of materials have been used as conduits, from nonresorbable materials such as silicone or GoreTex (12) to freeze-dried muscle grafts (13,14) to vein grafts, and have shown some good results, with vein graft conduits being shown to have significant return of sensation in gaps up to 5 mm (15). Resorbable conduits made of type I collagen, polyglycolic acid, or porcine intestinal submucosa (Figure 7) have become the latest and most commonly used materials for this purpose, and the conduit-assisted tensionless microsurgical nerve coaptation is associated with less sensory disturbances when compared with direct suture neurorrhaphy (16). A third-generation of conduits are currently under development, attempting to incorporate controlled delivery of neurotrophic growth factors, stem cells, and Schwann cells to aid in regeneration (17,18). Safa and Buncke’s review found that in gaps of less than 6mm, conduits will consistently achieve FNSR (19). Lohmeyer et al. performed a literature review of sensory recovery after digital nerve reconstructions using nerve conduits between 5 and 25 mm and found similar results, showing a significant decline in sensory recovery occurring in gaps larger than 6 mm when repaired with hollow conduits (20). Connectors, conduits, and nerve protectors or wraps also serve a significant purpose in isolating the region of neuronal regeneration from the surrounding wound bed as a barrier membrane, preventing interference from ingrowth of scar tissue, inflammation, as well as isolating the healing area from outside mechanical trauma.
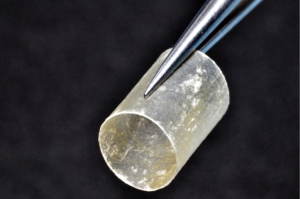
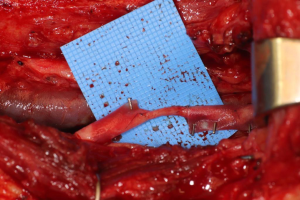
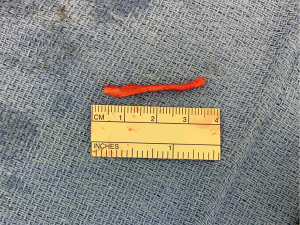
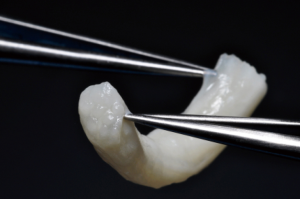
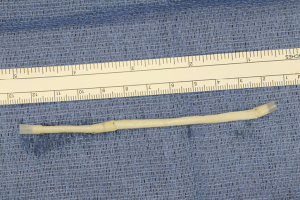
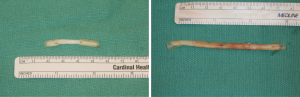
Finally, in cases of larger nerve resections (leaving more than 6 mm gap) interpositional or cable grafts become necessary to maximize the success rates of sensory recovery. Autogenous nerve grafts (21,22) have been used more often in the past, with the sural and greater auricular nerves (Figure 8) receiving the most attention in the head and neck reconstructive forum (23,24). However in recent years, nerve allografts have been increasingly used for this purpose and have shown some excellent results, with the release of processed nerve allograft (PNA—Figure 9) in 2008 (Avance; AxoGen: Alachua, Florida), beginning initially in extremity reconstruction and extending in the head and neck surgery realm, showing very similar results to autografts (25-27). Allografts allow for the preservation of nerve form and architecture, as well as the extracellular matrix microenvironment, promoting an optimized nerve regeneration scaffold. These allografts are both decellularized and sterilized, which significantly reduces the risk of immune rejection and thus eliminates the need for immunosuppressive therapy (28). In a review of the literature by Ducic and Yoon (29) found that allografts and autografts in the setting of nerve repair showed a statistically significant improvement in sensory recovery compared to direct or conduit-assisted repair, and additionally found that there was no difference in success rates between allografts and autografts, a significant finding, with allografts having several advantages over autografts. Avoiding a second surgical site for nerve graft harvest allows for decreased operating time, as well as avoids the morbidity of sensory deficits in the dermatome supplied by the harvested nerve; in the case of the greater auricular, a numb ear, in the case of the sural, a numb ankle/foot. Moreover, depending on the type of autogenous graft used, there may be a limitation of the length of graft that may be harvested, which may represent a limiting factor if a longer gap needs to be reconstructed. With PNA, however, up to 70 mm graft is available, which is approximately the average distance from the lingula to the mental foramen, and thus meets the needs of almost all situations, even in a situation where nerve sharing will be performed between the distal end of one branch of the trigeminal system with another branch, in which significant length may be needed. Additionally, nerve allografts can be sutured together consecutively in series to obtain increased length in cases of larger gaps (Figure 10). Nerve regeneration occurs at a rate of approximately 1 mm/day, however rates anywhere from 0.2–3 mm/day have been reported in the literature (30). As a technical note, the host nerve should always be prepared before the graft is selected, so that graft length can be determined accurately. Not only will the cut host nerve retract, yielding a larger defect, but the harvested nerve graft shrinks in length by approximately 20%, and additional length may be lost in final preparation of host and nerve graft ends. Therefore, the nerve graft harvested should be at least 25% longer than the host nerve defect to compensate for these changes (31). Moreover, there exists a diameter discrepancy between these commonly used nerve autografts and the LN and IAN, as the sural nerve is on average about 2.1 mm and the greater auricular is about 1.5 mm, both significantly smaller than the IAN and LN, whereas with allografts, several diameters are available to ideally match the size of the nerve being reconstructed (32). Matching the number of fascicles between host nerve and graft is also important, to optimize the patient’s postoperative return of sensation, and to minimize loss of fascicles and neuroma formation. The sural and greater auricular nerves contain only between 44% and 69% of the number of fascicles as compared to the IAN and LNs (33), as compared to allografts that can be more easily matched in terms of fascicle number. A landmark paper by Zuniga examined success rates of allograft reconstruction in patients with nerve defects from trauma (dentoalveolar, orthognathic) as well as in oncologic defects, and showed 87% and 88% FNSR in LN and IAN, respectively, showing 100% improvement if repair was performed within 90 days of injury (34). Brooks and colleagues looked at the FNSR in nerve gap reconstructions using PNA in small defects between 5 and 50 mm, and acceptable recovery was noted in 87% of patients with only a 5% revision rate (35). Oncologic nerve defects tend to be some of the longest-span defects, with gaps often spanning more than 50 mm due to the size of the pathology and the need for resection with adequate surgical margins, and have been shown to have similar FNSR as shown by Salomon et al., with 85.7% of patients showing at least S3 recovery, in cases of both benign as well as malignant pathology (36). Another study examined the results of immediate reconstruction of the IAN with PNA in resection of strictly benign pathology, with all nerve grafts exceeding 45 mm, with a total of 18 patients included in the study, and noted a 90% FNSR at 12 months postoperatively, with 44% achieving recovery after only 3 months (37).
Operative techniques
Surgical management of nerve defects is done in a methodical and stepwise fashion in order to optimize the technical aspects of repair as well as postoperative results. First the nerve must be carefully inspected and any nearby foreign bodies, bone, and scar tissue must be removed. Next, the health of each nerve stump must be assessed, examining for good intraneural capillary bleeding, properly aligned fascicles, and clean sharp tissue margins, resecting nerve tissue until these are seen. A study by Wolfe showed that success of nerve repair depends heavily on having healthy nerve endings, with at least 75% healthy fascicles needed for ideal repair (38). At this juncture, the nerve endings should be opposed in a tensionless manner, mobilization of nerve as needed, and determination of where the repaired nerve will lie in the tissues, to gauge which method of nerve repair is to be undertaken. If nerve stumps come in close proximity to each other with no tension, a direct neurorrhaphy (Figure 11) may be attempted with epineural interrupted sutures, generally 8-O nylon. Two sutures may be placed at 12 o’clock and 6 o’clock, or if additional sutures are desired, three can be placed at the 4, 8 and 12 o’clock positions, and care must be taken to only suture epineurium and not disturb, distort or injure the fascicles with the suture needle, as this can induce scarring and disrupt fascicular orientation. In addition to epineurial repair, interfascicular and fascicular (perineurial) repairs have been done in extremity nerve reconstruction, and though these methods allow for more direct fascicular alignment, they have been shown to have deleterious effects in regards to increased scarring and disruption in blood supply (39). In order to protect the coaptation site a nerve protector may be wrapped around the site of repair to serve as a barrier membrane, and this membrane may be secured with microclips placed perpendicular to the axis of the nerve. The use of direct neurorrhaphy, though still used occasionally, has been largely replaced by conduit-assisted repair, even in the case where no gap or tension exists, due to its increased predictability and protection of the coaptation site. If a larger gap exists (from 2–6 mm) or there is tension in directly opposing nerve endings, a conduit-assisted repair (Figure 12) can be used. In this situation, the conduit is placed around one of the free nerve ends and secured in place with two 8-O nylon interrupted epineurial sutures at 12 o’clock and 6 o’clock, and then the opposing nerve ending is fed into the connector and the same sutures are placed on the other side, while ensuring not to crush the nerve with forceps, handling the nerve only via the epineurium. The translucency of the intestinal submucosa nerve conduit is important for proper visualization of the connecting recipient nerve ending and allograft interface, ensuring optimal apposition and spacing. Other commonly used hollow tubes, like collagen, polyglycolic acid, and vein grafts lack the translucent advantages of a porcine submucosa connector.
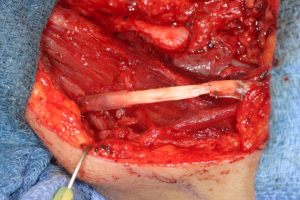
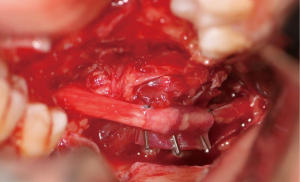
In cases where interpositional or cable grafts are necessary, the autogenous nerve graft or PNA are prepared on the back table and brought into the surgical field once the nerve-connector complex is assembled. If using allograft, the appropriate sized PNA is chosen, generally 3–4 mm for IAN and 3–5 mm for LN reconstruction, and clean sharp nerve ends must be established with a fresh scalpel blade, and the appropriate sized connector is chosen. A connector is placed around either nerve ending, ensuring to position the nerve edge where only a small 1–2 mm gap will exist with the native nerve stump. Two 8-O nylon interrupted epineurial sutures are placed at 12 o’clock and 6 o’clock positions, suturing from graft to connector for ease of passing the stitch. Once the connector-graft-connector unit is assembled (Figure 13), it is transferred to the surgical field, and coapted to the native nerve stumps with the same sequences of epineurial sutures. For ease of inset with increased access and visibility, the proximal end is coapted first, then the distal end, sometimes utilizing a “parachuting technique” (Figure 14). Simultaneous reconstruction of the IAN and LN is large ablative defects is also possible, the difficulty often being access to both proximal nerve stumps, however depending on the surgical approach, this can be accomplished (Figure 15).
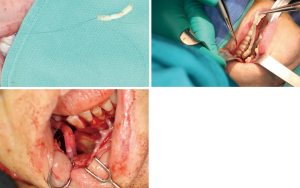
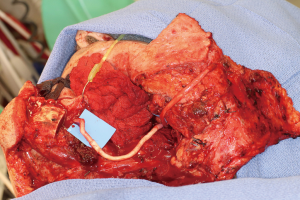
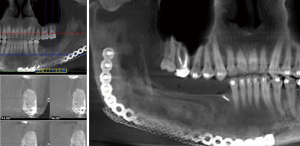
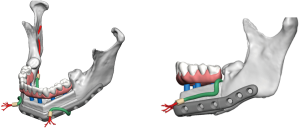
Positioning of the reconstructed nerve is vital to ensure its protection and to maximize its regeneration, remaining passive within the tissues and without outside impingement or risk of damage. With LN reconstruction, the position of the nerve is fairly constant, and remains in its native position, within the soft tissues of the floor of mouth. However with the reconstructed IAN, its final positioning within the tissues is dependent on several factors, including the type of mandibular reconstruction. If the mandibular discontinuity is to be reconstructed with only a plate with or without a soft tissue flap, then placement of the nerve passively within the soft tissues is all that is needed (Figure 16). If, however, bone is to be placed, the orientation and positioning of the reconstructed nerve is more challenging. If a non-vascularized bone graft is being placed, the nerve can be placed outside of the graft, or ideally within the bone graft, where a neo-mandibular canal can be formed with maturation of the graft (Figure 17), affording it added protection. If, however, a vascularized free osseous flap is being used for reconstruction, the position of the nerve must be carefully thought out, since it cannot be placed within the bone. The nerve can be positioned either under or over the bony segment as it passes from proximal to distal (Figure 18), however if dental implants are planned, placement under the bone is more acceptable, since the nerve may be damaged from above during the subsequent dental implant surgeries, unless the resection is more proximal and the most posterior implants are far from the resection margin. The final position of the nerve can be determined beforehand if VSP is to be employed pre-operatively during the planning of the free flap reconstruction (40) (Figure 19).
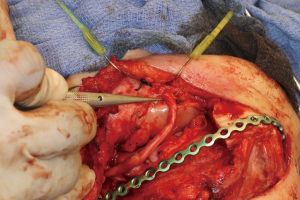
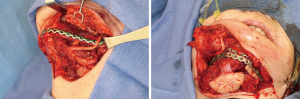
Recovery and rehabilitation
Postoperative neurosensory recovery is multifactorial, with many factors influencing the quality of the results achieved. The definition of a successful outcome varies significantly among surgeons and patients. It is important to gauge the patient’s expectations of sensory recovery before ever heading to the operating room, as even the best result may not restore function to the premorbid level, and patients should be made aware of that prior to surgery. For example, in LN repair, return of taste sensation should not be expected, though it may improve after reconstruction. The ability to reconstruct a nerve is only as viable as the number of successful outcomes, which are typically recorded as percentage of functional sensory recovery. There are a number of subjective methods to test for sensory recovery following nerve reconstruction including: soft touch perception, temperature perception, and pain perception. Mapping out the area of recovery on a patient over time allows for thorough tracking of progress and comparison to previous examinations. Objective evaluation of postoperative recovery is based on the Medical Research Council Scale adapted to the Oral and Maxillofacial region (Table 1) (41). Using this scale, a score of S3 or higher is generally accepted as having achieved FNSR. Sensory re-education has proven to be beneficial in the postoperative period once responses to pain and light touch have returned (42).
Table 1
| Grade | Description |
|---|---|
| S0 | No sensation |
| S1 | Recovery of deep cutaneous pain and tactile sensibility |
| S2 | Recovery of some superficial pain and touch |
| S2+ | Pain and touch sensation (S2) with hyperesthesia/overresponse |
| S3 | Pain and touch sensation (S2) without hyperesthesia/overresponse; static 2-point discrimination >15 mm |
| S3+ | As in S3 with static 2-point discrimination 7–15 mm |
| S4 | Normal sensation with 2-point discrimination between 2–6 mm |
Conclusions
Nerve reconstruction has gained significant ground recently in advancing the concept of “functional reconstruction” of head and neck ablative defects. Postoperative dysfunction of the trigeminal system in particular, can have a significant impact on physiologic function as well as psychosocial impairment, and restoration of nerve function can play an important role in improving the quality of life of patients. If possible, immediate microsurgical repair of sensory nerves should be undertaken during reconstruction of ablative defects, and should be done in a stepwise, methodical manner in order to increase chances of success and predictable outcome. There exist several techniques for nerve reconstruction, however the use of allografts has significantly improved results while decreasing the morbidity of autogenous grafting, and has helped in overcoming the shortfalls of primary and conduit-assisted repair. Nerve allografts can be used in both benign as well as malignant pathology, can reconstruct larger defects with excellent results, and are easily available for use. Head and neck oncologic and reconstructive surgeons should be aware of the predictability of nerve reconstruction techniques to optimize patients’ functional recovery in the postoperative period.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paolo Cariati) for the series “Microvascular reconstruction of head and neck oncological defects—state of the art” published in Frontiers of Oral and Maxillofacial Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form, available at: https://fomm.amegroups.org/article/view/10.21037/fomm-2020-mr-02/coif. The series “Microvascular reconstruction of head and neck oncological defects—state of the art” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pogrel MA, Schmidt BL, Sambajon V, et al. Lingual nerve damage due to inferior alveolar nerve blocks: a possible explanation. J Am Dent Assoc 2003;134:195-9. [Crossref] [PubMed]
- Svane TJ, Wolford LM, Milam SB, et al. Fascicular characteristics of the human inferior alveolar nerve. J Oral Maxillofac Surg 1986;44:431-4. [Crossref] [PubMed]
- Engelbrecht H, Shabnum M, Kourie J. Perineural infiltration of the inferior alveolar nerve in mandibular ameloblastomas. Br J Oral Maxillofac Surg 2013;51:757-61. [Crossref] [PubMed]
- Leung YY. Management and prevention of third molar surgery-related trigeminal nerve injury: time for a rethink. J Korean Assoc Oral Maxillofac Surg 2019;45:233-40. [Crossref] [PubMed]
- Leung YY, Lee TC, Ho SM, et al. Trigeminal neurosensory deficit and patient reported outcome measures: the effect on life satisfaction and depression symptoms. PLoS One 2013;8:e72891. [Crossref] [PubMed]
- Yi C, Dahlin LB. Impaired nerve regeneration and Schwann cell activation after repair with tension. Neuroreport 2010;21:958-62. [Crossref] [PubMed]
- Smith KG, Robinson PP. An experimental study of three methods of lingual nerve defect repair. J Oral Maxillofac Surg 1995;53:1052. [Crossref] [PubMed]
- Miyamoto Y. Experimental study of results of nerve suture under tension vs. nerve grafting. Plast Reconstr Surg 1979;64:540. [Crossref] [PubMed]
- Bagheri SC, Meyer RA, Cho SH, et al. Microsurgical repair of the inferior alveolar nerve: success rate and factors that adversely affect outcome. J Oral Maxillofac Surg 2012;70:1978-90. [Crossref] [PubMed]
- Mozsary PG, Middleton R. Microsurgical reconstruction of the lingual nerve. J Oral Maxillofac Surg 1984;42:415-20. [Crossref] [PubMed]
- Susarla SM, Kaban LB, Donoff RB, et al. Does early repair of lingual nerve injuries improve functional sensory recovery? J Oral Maxillofac Surg 2007;65:1070-6. [Crossref] [PubMed]
- Pogrel MA, McDonald AR, Kaban LB. Gore-Tex tubing as a conduit for repair of lingual and inferior alveolar nerve continuity defects: A preliminary report. J Oral Maxillofac Surg 1998;56:319. [Crossref] [PubMed]
- Feneley MR, Fawcett JW, Keynes RJ. The role of Schwann cells in the regeneration of peripheral nerve through basal muscle grafts. Exp Neurol 1991;114:275. [Crossref] [PubMed]
- DeFranzo AJ, Morykwas MJ, LaRosse JR. Autologous denatured muscle as a nerve graft. J Reconstr Microsurg 1994;10:145. [Crossref] [PubMed]
- Pogrel MA, Aziz M. The Use of Autogenous Vein Grafts for Inferior Alveolar and Lingual Nerve Reconstruction. J Oral Maxillofac Surg 2001;59:985-8. [Crossref] [PubMed]
- Ducic I, Safa B, DeVinney E. Refinements of nerve repair with connector-assisted coaptation. Microsurgery 2017;37:256-63. [Crossref] [PubMed]
- Gaudin R, Knipfer C, Henningsen A, et al. Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Res Int 2016;2016:3856262. [Crossref] [PubMed]
- Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am 2000;25:391-414. [Crossref] [PubMed]
- Safa B, Buncke G. Autograft substitutes: conduits and processed nerve allografts. Hand Clin 2016;32:127-40. [Crossref] [PubMed]
- Lohmeyer JA, Kern Y, Schmauss D, et al. Prospective clinical study on digital nerve repair with collagen nerve conduits and review of literature. J Reconstr Microsurg 2014;30:227-34. [PubMed]
- Miloro M, Stoner JA. Subjective outcomes following sural nerve harvest. J Oral Maxillofac Surg 2005;63:1150. [Crossref] [PubMed]
- Bagheri SC, Meyer RA, Khan HA, et al. Retrospective review of microsurgical repair of 222 lingual nerve injuries. J Oral Maxillofac Surg 2010;68:715. [Crossref] [PubMed]
- LaBanc JP, Epker BN. Trigeminal nerve microreconstructive surgery using the greater auricular nerve transfer technique. Oral Maxillofac Surg Clin North Am 1992;4:459.
- McCormick SU, Buchbinder D, McCormick SA, et al. Microanatomic analysis of the medial antebrachial nerve as a potential donor nerve in maxillofacial grafting. J Oral Maxillofac Surg 1994;52:1022. [Crossref] [PubMed]
- Cho MS, Rinker BD, Weber RV, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am 2012;37:2340-9. [Crossref] [PubMed]
- Shanti RM, Ziccardi VB. Use of decellularized nerve allograft for inferior alveolar nerve reconstruction: A case report. J Oral Maxillofac Surg 2011;69:550. [Crossref] [PubMed]
- Tang P, Whiteman DR, Voigt C, et al. No Difference in Outcomes Detected Between Decellular Nerve Allograft and Cable Autograft in Rat Sciatic Nerve Defects. J Bone Joint Surg Am 2019;101:e42. [Crossref] [PubMed]
- Whitlock EL, Tuffaha SH, Luciano JP, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 2009;39:787-99. [Crossref] [PubMed]
- Ducic I, Yoon J. Reconstructive Options for Inferior Alveolar and Lingual Nerve Injuries After Dental and Oral Surgery An Evidence-Based Review. Ann Plast Surg 2019;82:653-60. [Crossref] [PubMed]
- Chiu DT, Strauch B. A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast Reconstr Surg 1990;86:928-34. [Crossref] [PubMed]
- Mackinnon SE, Dellon AL. Surgery of the peripheral nerve. New York: Thieme; 1988.
- Brammer JP, Epker BN. Anatomic-histologic survey of the sural nerve: Implications for inferior alveolar nerve grafting. J Oral Maxillofac Surg 1988;46:111-7. [Crossref] [PubMed]
- Svane TJ. The fascicular characteristics of human inferior alveolar and greater auricular nerves [master’s thesis]. Waco (TX): Baylor University; 1989.
- Zuniga JR. Sensory Outcomes After Reconstruction of Lingual and Inferior Alveolar Nerve Discontinuities Using Processed Nerve Allograft - A Case Series. J Oral Maxillofac Surg 2015;73:734-44. [Crossref] [PubMed]
- Brooks DN, Weber RV, Chao JD, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstruction. Microsurgery 2012;32:1-14. [Crossref] [PubMed]
- Salomon D, Miloro M, Kolokythas A. Outcomes of Immediate Allograft Reconstruction of Long-Span Defects of the Inferior Alveolar Nerve. J Oral Maxillofac Surg 2016;74:2507-14. [Crossref] [PubMed]
- Zuniga JR, Williams F, Petrisor D A. Case-and-Control, Multisite, Positive Controlled, Prospective Study of the Safety and Effectiveness of Immediate Inferior Alveolar Nerve Processed Nerve Allograft Reconstruction With Ablation of the Mandible for Benign Pathology. J Oral Maxillofac Surg 2017;75:2669-81. [Crossref] [PubMed]
- Wolfe SW, Johnsen PH, Lee SK, et al. Long-Nerve Grafts and Nerve Transfers Demonstrate Comparable Outcomes for Axillary Nerve Injuries. J Hand Surg Am 2014;39:1351-7. [Crossref] [PubMed]
- Levinthal R, Brown WJ, Rand RW. Comparison of fascicular, interfascicular and epineural suture techniques in the repair of simple nerve lacerations. J Neurosurg 1977;47:744-50. [Crossref] [PubMed]
- Miloro M, Markiewicz MR. Virtual Surgical Planning for Inferior Alveolar Nerve Reconstruction. J Oral Maxillofac Surg 2017;75:2442-8. [Crossref] [PubMed]
- Meyer RA, Rath EM. Sensory rehabilitation after trigeminal nerve injury or nerve repair. Oral Maxillofac Surg Clin N Am 2001;13:365-76.
- Phillips C, Blakey G 3rd, Essick GK. Sensory retraining: a cognitive behavioral therapy for altered sensation. Atlas Oral Maxillofac Surg Clin North Am 2011;19:109-8. [Crossref] [PubMed]
Cite this article as: Kaleem A, Patel N, Geiger JF 3rd, Tursun R. Processed nerve allografts in reconstructive microneurosurgery after ablative head and neck surgery: an overview. Front Oral Maxillofac Med 2020;2:16.