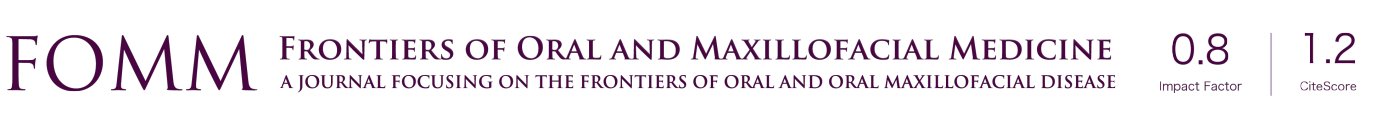Acoustic emissions of the temporomandibular joint in children: proof of concept
Introduction
Assessment of the temporomandibular joint (TMJ) can be difficult; clinical signs and symptoms are non-specific (1), examination is challenging and imaging is often necessary (2,3). TMJ disease in children can cause pain and growth disturbances leading to malocclusion and/or skeletal deformities (2,4). The presentation, difficulty in diagnosis, and severity of sequelae of untreated disease present a compelling need for the development of a biomarker for TMJ health (2). Ideally, this biomarker would be objective, noninvasive, and readily measurable with affordable hardware. Acoustic emissions (AEs) from the TMJ could serve as such a biomarker. AEs are the sounds produced during joint articulation. They contain information related to the structural integrity of the joint and the health of internal articulating surfaces (5,6). Changes to AEs could serve as an objective diagnostic method of TMJ pathology.
AEs from joints were first reported in 1902 by Blodgett (7). In the 1930s, Steindler correlated joint malfunctions and sounds using several types of sound detecting equipment (8). In 1961, Brackin filed the first patent detailing an apparatus for recording and analyzing joint disorders with unique acoustic patterns recorded from different pathologies (9). These attempts to facilitate diagnostic procedures by microphonic detection of emissions did not gain widespread use because of discrepancy in the nature of the sounds and the recording technique (10). In 1984, Mollan found that the use of a piezoelectric accelerometer detector in direct contact with the skin gave a robust signal and allowed for detection in the subsonic frequency range (10). Five years later, Gay filed a patent for a diagnostic procedure and apparatus that quantitatively correlated joint-induced sound patterns relative to the joint position in time, and noted that it could be particularly useful in diagnosing TMJ disorders (11). Gay’s technique was the first to move away from qualitative descriptors of the joint sounds to quantitatively compare the sound profiles.
Prior to the 1990’s, joint AE analysis was limited by the computational power and by the physical size of the sensors, so research focused on larger, more accessible joints (e.g., the knee). As a result of those limitations, comparisons were often qualitative and inconsistent between researchers. The advent of miniaturized sensors and the increasing computational power of the 1990’s presented the opportunity for more powerful (and quantitative) AE analysis of smaller joints (e.g., the TMJ). Since then, two main approaches for recording TMJ AEs have gained prevalence in the field: binaural miniature microphones placed at the intra-auditory meatus and contact accelerometers placed on bony prominences around the joint (12). Microphones at the intra-auditory meatus provide a broad signal-to-noise bandwidth, while contact accelerometers provide the highest mean amplitude in the time domain waveform (13). Either approach is suitable depending on the application and nature of the underlying signal being recorded. In this project, surface mounted accelerometers were used because they are easy to place on TMJs and are able to capture high amplitude spikes in the AEs.
Significant steps have been made in the quantitative classification of these audio signals. Prinz showed that the time domain is where most of the characteristic differences of the various TMJ AEs are found, and that the frequency domain was much less distinct than the time domain (14). To study key signal features in the time domain, several computationally rigorous approaches have been applied such as the reduced interference-distribution (RID) of the time-frequency energy distributions and neural networks. The RID technique was shown to have a more detailed classification of TMJ AEs than by auscultation (15-17). Neural networks were used to classify TMJ sounds based on their narrow-band, wide-band, and time-varying frequency components (18). Previous research on TMJ AEs resulted in several patents for devices which capture TMJ AEs. However, this type of analysis has not gained widespread clinical usage perhaps because a standard protocol for best capturing and analyzing AEs does not exist.
AEs of TMJs must be better understood, characterized and a standardized technique for recording and interpretation needs to be developed. The purposes of this project were to: (I) present a custom, wearable headset with embedded contact accelerometers and (II) assess the repeatability and reliability of four headset in children. We hypothesize that this headset will allow for the convenient recording of AEs, which will ultimately facilitate their inclusion as a biomarker in a clinical workup of the TMJ. The work presented here is an early, but crucial step toward the design of a system for augmenting the current diagnosis and monitoring of TMJ disease in children.
Methods
Subject recruitment
Institutional Review Board approval was obtained (#00081670), and all subjects were recruited in accordance with the Helsinki Declaration guidelines. Inclusion criteria consisted of children 6–18 years of age who presented to the Oral and Maxillofacial Surgery (OMS) clinic for a non-TMJ related reason. The presence or absence of TMJ sounds was verified via a clinical examination by a board-certified OMS. Exclusion criteria were: (I) systemic disease which has potential to affect the TMJ [e.g., juvenile idiopathic arthritis (JIA)], (II) history of craniofacial syndromes with potential for TMJ involvement (e.g., hemifacial microsomia), (III) history of TMJ/facial trauma, (IV) ongoing orthodontics, (V) history of any surgery to the TMJ or surrounding areas, and/or (VI) complaints of TMJ dysfunction (TMD).
Device/headset setup
When a subject opens and closes his/her jaw, TMJ articulation creates vibrations that are detectable on the surface of the skin. The headset was built to be adjustable and to fit 95% of users younger than 18 years old based on anthropometric head circumference data (19). The headset is positioned on the subjects’ heads with skin contact accelerometers against the articular eminences of TMJs (Figure 1) (20,21). This location and skin contact previously demonstrated detection of TMJ sounds with the highest quality time domain waveforms (13). This method provided sufficient contact force without hindering portability of the device or causing discomfort (12).
The AEs were recorded using Dytran uniaxial, miniature accelerometers (Model 3225F7, Dytran Inc., Chatsworth, CA 91311, USA) with a diameter of 6.35 mm. They are highly sensitive to acceleration (sensitivity is 10.2 mV/m/s2) and the frequency response curve is flat from 2 Hz to 10 kHz. The accelerometers were connected to a data acquisition device that enables the simultaneous and synchronous capture of bilateral accelerometers at a sampling rate of 100 kHz. The sensors and the data acquisition device are plugged into a laptop that powers the devices and is running a custom program written in MATLAB (Figure 1C). This program controls the length of the recording and converts the voltage readouts from the sensors to units of acceleration (using the manufacturer-provided calibrated sensitivities of the specific accelerometers). The program also performs preliminary steps to ensure that the data are successfully recorded including bandpass filtering (between 250 Hz–20 kHz) and plotting the recordings. This filtering range isolates the frequencies containing the majority of TMJ AE signals, and removes artifacts associated with large-scale movement of the jaw, low frequency muscle sounds, and environmental noise (12,14,22). With the setup in place and the software running, the subjects perform 10 repetitions of opening/closing their mouth at a rate of 1 repetition every 4 seconds (Figure 1). The raw and filtered data were recorded and locally stored for further processing.
Feasibility and repeatability
To assess the feasibility of using the TMJ AE recording headset, AEs from one healthy control (i.e., no TMJ sounds) and one patient with clinically noticeable TMJ sounds were recorded. These recordings were qualitatively compared to ensure that there were differences in the sounds and that the headset was recording AEs properly. To assess the repeatability of the recording device, 9 subjects performed three trials of open/close movements while their AEs were recorded. Between each trial, the headset was removed and repositioned on the subject’s head to test for repeatability of the placement of the device.
Analysis
To analyze repeatability of measurements from the AEs of TMJs, three features that describe the signals were calculated: the root mean square (RMS) amplitude, the signal energy, and the zero-crossing rate (ZCR). RMS is essentially a measure of the absolute value of the magnitude of the signal, so signals with larger spikes would be expected to have a larger RMS amplitude. The energy feature is computed as the integral of the squared signal magnitude. This feature describes how “loud” the audio signal is. The ZCR describes how often the signal crosses zero, which estimates how quickly its values change. The ZCR is used to quantify how often the signal is moving from negative to positive and back indicating a change in direction as the skin vibrates. If the skin was vibrating back-and-forth faster, then the ZCR would increase. Together, these three features comprehensively describe the observed qualitative differences.
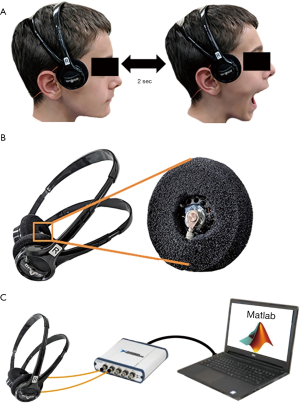
Repeatability of measurements on each subject was calculated using the intra-class correlation coefficient (ICC). The ICC indicates how strongly the different sessions of TMJ recordings resemble each other. The ICC varies from 0 to 1 (1 indicates completely the same, 0 indicates no overlap) with values above 0.9 typically representing excellent repeatability (23). The ICC was calculated for each of the features selected to describe the signal (i.e., the RMS amplitude, energy and ZCR) for all trials. Each TMJ (left and right) of a patient was a separate group. This was done because the goal of this analysis was to ensure that the device was recording a repeatable signal from each specific TMJ, not to make any claims of population-wide AE patterns. There is inherent inter-subject and intra-subject variability in the AEs of each TMJ since each individual TMJ has unique anatomy and kinematics (Figure 2).
Results
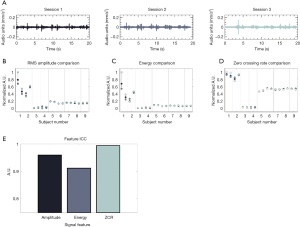
To test the headset’s recording capabilities, recordings were obtained to ensure the device was working properly. Sounds were recorded from TMJs of a healthy subject with TMJ sounds and sounds from TMJs of a healthy subject without TMJ sounds. There are several qualitative differences between the two subjects’ recordings (Figure 3). The patient with sounds had large spikes (with amplitudes of ~0.1 mm/s2) that occurred approximately every four seconds (Figure 3B), These spikes sounded like loud clicks or pops when listening to the recordings. These sounds were occurring at the same point in the articulation of the jaw during each cycle of opening and closing. In addition, the TMJ sounds were more heterogenous and variable than the ones from the child without TMJ sounds. The child without TMJ sounds had numerous smaller spikes in the sound (with magnitudes of ~0.5 mm/s2). When listening to these smaller spikes, they resembled a grinding sound.
Next, nine healthy children (6 females, 3 males) with mean age of 10.8±3.2 years (range, 7 to 16 years) had their AEs recorded in order to assess the repeatability of TMJ AE recordings. The three signal features discussed above (RMS amplitude, energy, and ZCR) were calculated for each of the three recordings from each TMJ on all the subjects. The goal of this analysis was to quantify how similar the signals from each recording sessions were for each subject. A representative example of the three recording trials for one subject can be seen in Figure 2A. The distribution of feature values across all the recording sessions and subjects can be seen in Figure 2B,C,D. Of note, though the individual feature values vary from subject to subject, the three sessions’ features were tightly clustered for each individual TMJ for all subjects. This tight clustering of feature values indicated that the signals were repeatable. To further quantify this repeatability, the ICC values are presented in Figure 2E. The ICC values were 0.96 for the RMS feature, 0.91 for the energy feature, and 0.995 for the ZCR feature. As discussed above, an ICC score >0.9 is considered to represent excellent similarity of the signals being assessed. Here, it indicated that the AE recordings are highly consistent across multiple recording sessions and placements of the headset.
Discussion
TMJ health is evaluated by a combination of physical exams and imaging studies. Physical exams rely on health care worker expertise. Imaging is not always feasible due to its high cost, need for occasional sedation in children, length of time, need for specialized equipment and potential contraindications (2,24). A TMJ AE headset has the potential to serve as a screening tool prior to obtaining imaging. The purposes of this manuscript were to (I) present a custom, wearable headset used to record AEs of TMJs, and (II) assess the repeatability and reliability of this headset in children.
The technique for measuring TMJ AEs has evolved since it was first proposed in 1902 (7). The field has progressed from manual auscultation, digital stethoscopes, condenser microphones, electret microphones, and now favors miniaturized contact accelerometers (9-13,25,26). The headset is based on findings of earlier work in selecting an ideal accelerometer with high sensitivity, and a bandpass filter to remove confounding low frequency muscle sounds and environmental noise (27). It was designed to obtain the highest amplitude signal in the time domain—which contains the majority of the characteristic differences in TMJ AEs (14). The device places the accelerometers superficial to the TMJ (12) and was designed specifically for children who are likely to be uncomfortable with an intra-aural device. This sensor location and comfortable form-factor minimized the time required to place the sensors accurately and firmly on the TMJs. The acquisition software was written to minimize computational time. Together, the form-factor, hardware, and recording scripts allowed for reproducible recordings of TMJ AEs with minimal time required for setup and acquisition (<2 minutes). Minimizing the time needed to assess the joint is of critical importance for a busy clinical setting.
Before exploring the diagnostic capabilities of the headset, the repeatability of the recordings needed to be quantified. To quantify this repeatability, three time-domain signal features were calculated: the RMS amplitude, ZCR, and energy. It was previously shown that time-domain features contained nearly all of the characteristic differences of TMJ AEs (14). In particular, the energy of the signal has been used extensively to describe characteristics of TMJ AEs (16,18). In the study, the ICC values were all >0.9, which indicated high consistency from one recording session to the next; thus, excellent repeatability (Figure 2). These findings support the claim that this wearable headset can consistently record AEs from the TMJ of children.
The sounds occurred at the same point in the articulation of the jaw during each cycle of opening and closing. We hypothesized that the cyclical occurrence of these loud sounds may indicate that there is an anatomical variation producing them. The TMJ sounds produced by the patient without clinically-evident sounds may simply indicate friction of the TMJ during articulation.
This study has a few limitations. Although the headset was removed multiple times, AEs were recorded during the same visit. This study shows that TMJ AEs can be successfully and repeatedly captured by a wearable headset. However, it does not address the variability in sounds overtime as disease progresses. Additionally, all the subjects recorded in this study were healthy with no history of TMJ dysfunction. This resulted in relatively small AEs, since the TMJs of healthy children are not expected to produce much sound. In the future, to better understand the feasibility of this technology for clinical diagnosis, it will be applied to children with systemic disease known to affect TMJ such as JIA and may be compared to MRI findings. This is the subject of an ongoing investigation in the center.
In conclusion, this project provides the foundation for the eventual clinical use of a TMJ AE device. In the future, this technology will be used on a cohort of patients with JIA and age/sex matched healthy controls to evaluate the effect of arthritis on the AEs of TMJs. In a chronic condition such as JIA, AE assessment may extend beyond just screening/diagnostics and instead be used as a longitudinal biomarker of disease activity within the joint. Overall, these exciting preliminary results should inspire further research into the acquisition, analysis, and classification of TMJ AEs.
Acknowledgments
The authors acknowledge the support of Lori Ponder.
Funding: This work was supported in part by the National Science Foundation under grant number 1749677.
Footnote
Peer Review File: Available at https://fomm.amegroups.org/article/view/10.21037/fomm-20-10/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://fomm.amegroups.org/article/view/10.21037/fomm-20-10/coif). Dr. Prahalad is supported by The Marcus Foundation Inc., Atlanta. Dr. Prahalad also serves on a Macrophage Activation Syndrome Advisory Committee for Novartis pharmaceuticals and participated in an advisory committee for Sigilon Therapeutics. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Institutional Review Board approval was obtained (#00081670), and all subjects were recruited in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cannizzaro E, Schroeder S, Müller LM, et al. Temporomandibular Joint Involvement in Children with Juvenile Idiopathic Arthritis. J Rheumatol 2011;38:510-5. [Crossref] [PubMed]
- Larheim TA, Doria AS, Kirkhus E, et al. TMJ imaging in JIA patients—An overview. Semin Orthod 2015;21:102-10. [Crossref]
- Abramowicz S, Kim S, Prahalad S, et al. Juvenile arthritis: current concepts in terminology, etiopathogenesis, diagnosis, and management. Int J Oral Maxillofac Surg 2016;45:801-12. [Crossref] [PubMed]
- Abramowicz S, Susarla HK, Kim S, et al. Physical Findings Associated With Active Temporomandibular Joint Inflammation in Children With Juvenile Idiopathic Arthritis. J Oral Maxillofac Surg 2013;71:1683-7. [Crossref] [PubMed]
- Inan OT, Whittingslow DC, Teague CN, et al. Wearable knee health system employing novel physiological biomarkers. J Appl Physiol 2018;124:537-47. [Crossref] [PubMed]
- Whittingslow DC, Jeong HK, Ganti VG, et al. Acoustic Emissions as a Non-invasive Biomarker of the Structural Health of the Knee. Ann Biomed Eng 2020;48:225-35. [Crossref] [PubMed]
- Blodgett WE. Auscultation of the Knee Joint. Bost Med Surg J 1902;146:63-6. [Crossref]
- Steindler A. Auscultation of Joints. J Bone Jt Surg 1937;19:121-36.
- Brackin R, Road S. Process and Apparatus for Analyzing Joint Disorders. US3181528. United States, 1961.
- Mollan R. Orthopedic Diagnostic Procedures and Apparatus Therefor. US4,437,437. United States, 1984.
- Gay T, Charles N. Bertolami DJS. Method and Apparatus for the Acoustic Detection and Analysis of Joint Disorders. US4,836,218. United States Patent, 1989.
- Łyżwa P, Kłaczyński M, Kazana P. Vibroacoustic Methods of Imaging in Selected Temporomandibular Joint Disorders During Movement. Diagnostyka 2018;19:109-17. [Crossref]
- Yoshida H, Sano T, Kataoka R, et al. A Preliminary Investigation of a Method of Detecting Temporomandibular Joint Sounds. J Orofac Pain 1994;8:73-9. [PubMed]
- Prinz JF, Ng KW. Characterization of Sounds Emanating from the Human Temporomandibular Joint. Arch Oral Biol 1996;41:631-9. [Crossref] [PubMed]
- Widmalm SE, Williams WJ, Zheng C. Time frequency distributions of TMJ sounds. J Oral Rehabil 1991;18:403-12. [Crossref] [PubMed]
- Widmalm SE, Williams WJ, Christiansen RL, et al. Classification of temporomandibular joint sounds based upon their reduced interference distribution. J Oral Rehabil 1996;23:35-43. [Crossref] [PubMed]
- Widmalm SE, Dong Y, Li BX, et al. Unbalanced lateral mandibular deviation associated with TMJ sound as a sign in TMJ disc dysfunction diagnosis. J Oral Rehabil 2016;43:911-20. [Crossref] [PubMed]
- Akan A, Başar Ünsal R. Time-frequency analysis and classification of temporomandibular joint sounds. J Franklin Inst 2000;337:437-51. [Crossref]
- de Onis M, Onyango AW, Van den Broeck J, et al. Measurement and Standardization Protocols for Anthropometry Used in the Construction of a New International Growth Reference. Food Nutr Bull 2004;25:S27-S36. [Crossref] [PubMed]
- Farkas LG, Posnick JC, Hreczko TM. Anthropometric Growth Study of the Head. Cleft Palate Craniofac J 1992;29:303-8. [Crossref] [PubMed]
- World Health Organization. WHO Child Growth Standards - Methods and development. 2007.
- Semiz B, Hersek S, Whittingslow DC, et al. Using Knee Acoustical Emissions for Sensing Joint Health in Patients with Juvenile Idiopathic Arthritis: A Pilot Study. IEEE Sens J 2018;18:9128-36. [Crossref] [PubMed]
- Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016;15:155-63. [Crossref] [PubMed]
- Petersson A. What you can and cannot see in TMJ imaging - an overview related to the RDC/TMD diagnostic system. J Oral Rehabil 2010;37:771-8. [Crossref] [PubMed]
- Chu ML, Gradisar IA, Railey MR, et al. Detection of knee joint diseases using acoustical pattern recognition technique. J Biomech 1976;9:111-4. [Crossref] [PubMed]
- Frank CB, Rangayyan RM, Bell GD. Analysis of knee joint sound signals for non-invasive diagnosis of cartilage pathology. IEEE Eng Med Biol Mag 1990;9:65-8. [Crossref] [PubMed]
- Oster G, Jaffe JS. Low Frequency Sounds from Sustained Contraction of Human Skeletal Muscle. Biophys J 1980;30:119-27. [Crossref] [PubMed]
Cite this article as: Whittingslow DC, Orlandic L, Gergely T, Prahalad S, Inan OT, Abramowicz S. Acoustic emissions of the temporomandibular joint in children: proof of concept. Front Oral Maxillofac Med 2020;2:10.